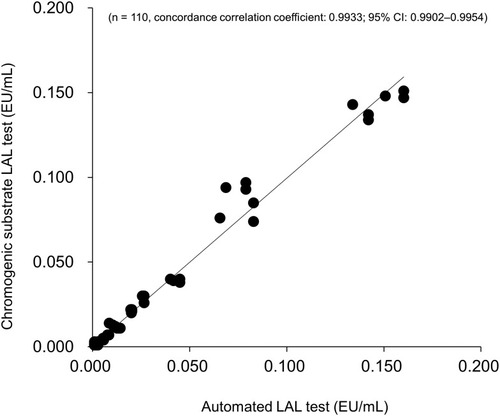
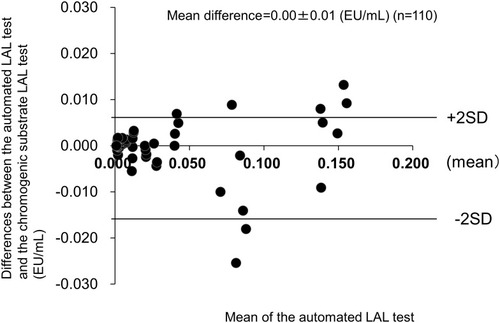
Figures & data
Figure 1 (A) Automatic LAL test device (Toxinometer ET-mini™). The device measures 27.7 cm (length)×17.6 cm (width)×8.5 cm (height), with a total weight of 2.4 kg. (B) The Toxinometer ET-mini can simultaneously measure four samples.
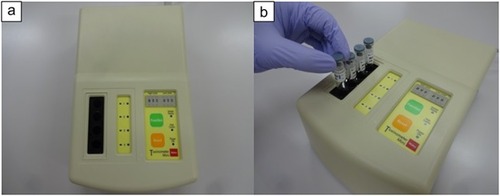
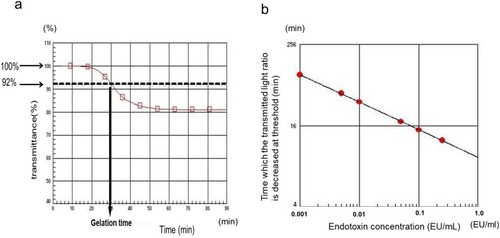
Figure 2 Principles of endotoxin activity measurement using the automatic LAL test (Toxinometer ET-mini™). (A) Endotoxin activity is determined from the time required for the transmitted light ratio to decrease below a certain threshold (92% of the initial value), which is defined as the gelation time. (B) The log value of endotoxin activity was calculated from the log–log plot of gelation time.