Figures & data
Table 1 Qualitative and Quantitative Preference Methods
Table 2 Advantages and Disadvantages of Preference TestingCitation1
Table 3 FDA Recommended Qualities for Patient Preference Testing
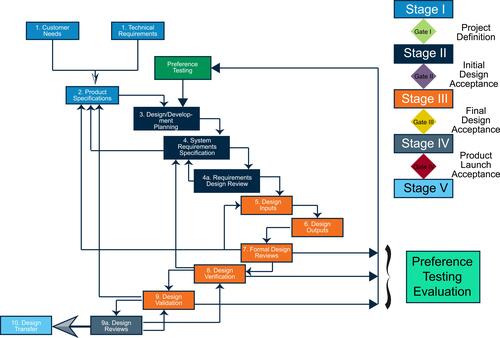
Figure 2 Stage-gate design concept for medical devices, including the conduction of preference testing. Data from sources.Citation22,Citation51