Figures & data
Figure 1 Categorization of bone-conduction devices.
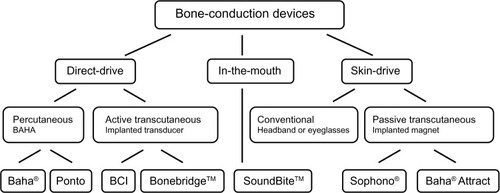
Figure 2 Conventional skin-drive bone-conduction devices, attached with (A) a steel spring headband, and (B) with frames for glasses.
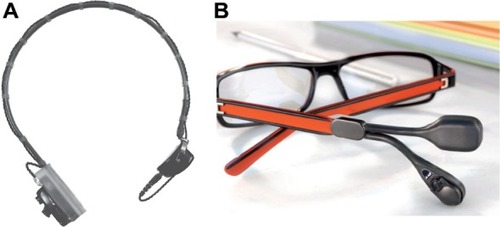
Figure 3 Sophono®, a passive transcutaneous skin-drive bone-conduction device.

Table 1 Summary of audiometric data of implantable bone conduction devices in clinical studies, only including studies that are reasonably comparable
Figure 4 Baha® Attract, a passive transcutaneous skin-drive bone-conduction device.

Figure 5 Bone-anchored hearing aid, a percutaneous direct-drive bone-conduction device.
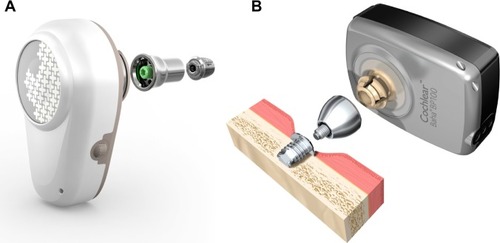
Figure 7 Bonebridge™, an active transcutaneous direct-drive bone-conduction device.

Figure 8 SoundBite™, an in-the-mouth bone-conduction device with implant for tooth attachment and behind-the-ear sound processor.

Table 2 MPO and reference thresholds for implantable BCDs
Table 3 Maximum dynamic ranges and suggested BC thresholds for implantable BC devices
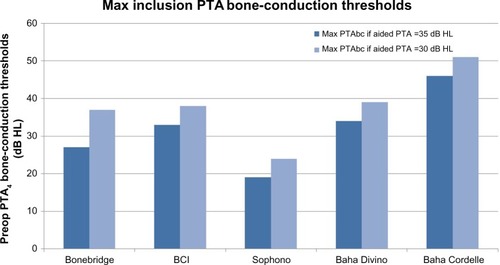
Figure 9 Estimated maximum recommended preoperative bone-conduction thresholds, which include a “gray” zone depending on if an aided PTA of at least 30 or 35 dB HL is met.