Figures & data
Table 1 Respondent Profiles (n = 102)
Figure 1 Overall receptivity to RebiSmart 3.0. †Indicates significantly higher than patients at 90% confidence.
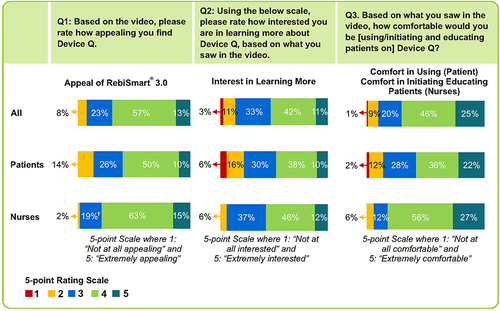
Figure 2 Importance of device features to user satisfaction: Anchored Probability Index. *Indicates significantly higher than patients at 90% confidence. †Indicates significantly higher than nurses at 90% confidence. ‡The anchored maximum difference analysis was used for the relative ranking of the device features from greatest to least importance to user satisfaction. §This question was asked multiple times and only 4 features were shown at a time and were randomized in each screenshot; so the respondent would end up seeing a single feature listed on different screenshots.
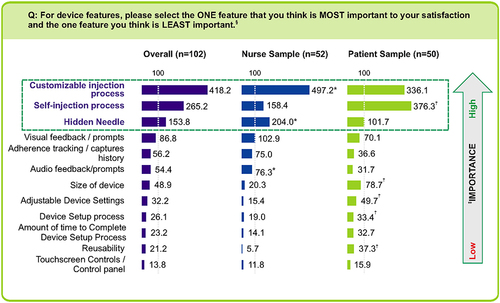
Figure 3 Performance of RebiSmart 3.0 vs other self-injecting devices on key features. *Indicates significantly higher than patients at 90% confidence. †RebiSmart® 2.0 Non-Users (n=23) that responded to questions on “Other devices performance”, were asked to consider the assistive device that they are currently using or recently used. ‡The anchored maximum difference analysis was used for the relative ranking of the device features from greatest to least importance to user satisfaction.
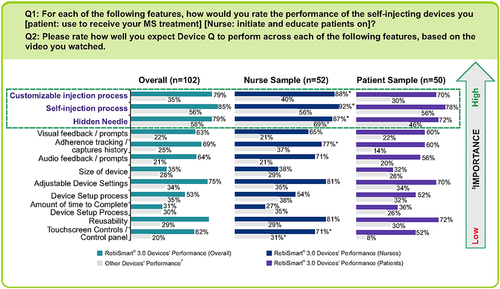
Figure 4 Performance of RebiSmart 3.0 by RebiSmart 2.0 users vs RebiSmart 2.0 non-users. Data refers to proportion of respondents rating the feature either very good (4) or excellent (5) on the 5-point scale. *Indicates significantly higher than RebiSmart® 2.0 device Non-Users at 90% confidence. †The anchored maximum difference analysis was used for the relative ranking of the device features from greatest to least importance to user satisfaction.
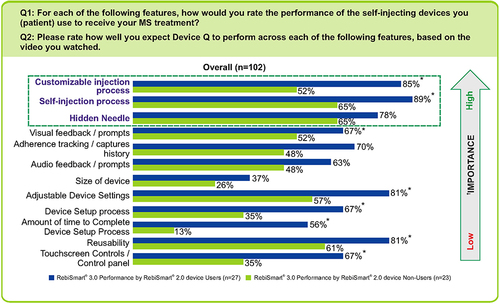
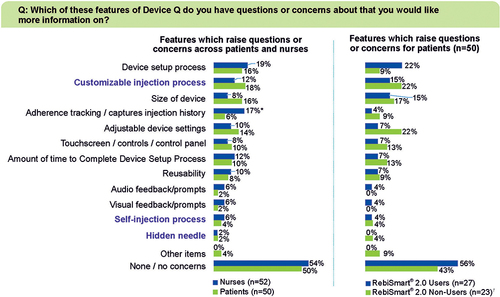
Figure 5 Features Which Raise Questions for Respondents (MS nurses and pwMS). *Indicates significantly higher than patients at 90% confidence. †RebiSmart® 2.0 Non-Users patients (n=23) that responded to questions on “Other devices performance”, were asked to consider the assistive device that they are currently using or recently used. Other devices included Avonex® pen and pre-filled syringe, Betaseron®/Betaferon® pre-filled syringe, ExtaviPro® 30G Autoinjector, Ypsomate®, glatiramer acetate pre-filled syringe and Plegridy® pen.