Figures & data
Table 1 Characteristics of devices and growth hormone formulations included in the analysis
Table 2 Example procedure for measuring growth hormone wastage with NordiPen®/SimpleXx® 5 mg or Omnitrope® Pen-5
Table 3 Laboratory analysis findings for 5 mg devices including dripping with the needle attached and when setting the GH dose, reported in mg of active GH (converted from mass of GH solution)
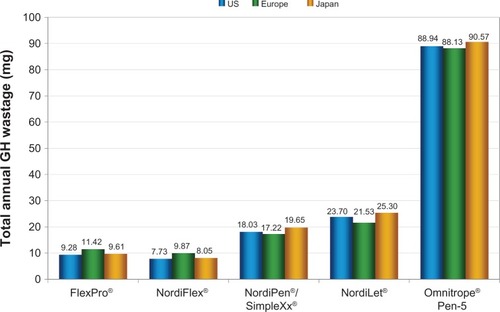
Figure 1 Modeled growth hormone wastage per patient per year for 5 mg administration devices in US, European, and Japanese settings.
Abbreviation: GH, growth hormone.
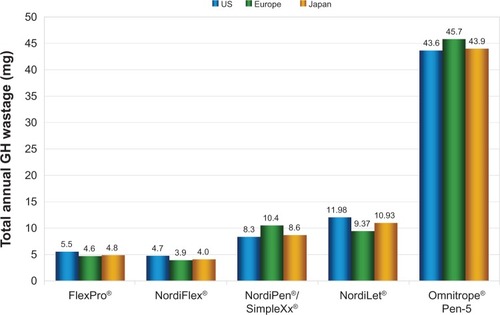
Table 4 Modeled annual GH wastage per patient with 5 mg and 10 mg devices in simulated US, European, and Japanese cohorts of pediatric patients on GH treatment
Figure 2 Growth hormone wastage per patient per year for 10 mg administration devices in US, European, and Japanese settings.
Abbreviation: GH, growth hormone.