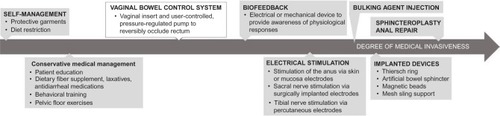
Figures & data
Figure 1 The vaginal bowel control (VBC) insert.
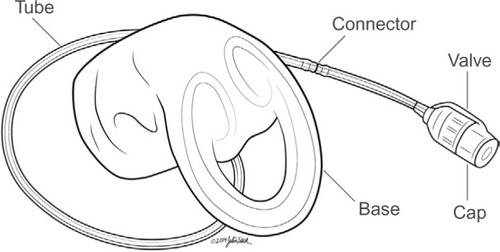
Figure 2 The vaginal bowel control insert deflated (A) and inflated (B).
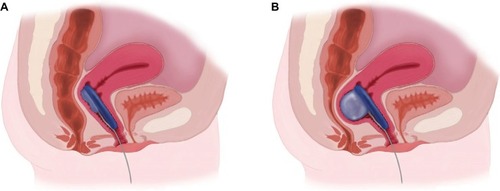
Figure 3 Folding the vaginal bowel control insert for placement.
Figure 5 Updated vaginal bowel control system that enhances fit, stability, comfort, and appearance based on the feasibility studies (courtesy of Pelvalon).
Figure 6 Within-subject efficacy of vaginal bowel control system wear on accidental bowel leakage (ABL) episodes.
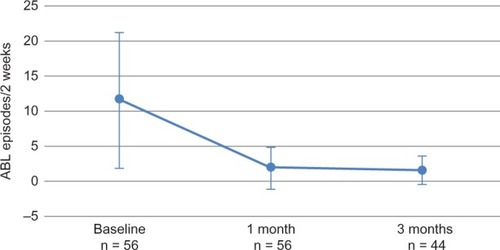
Table 1 Overview of a clinical evaluation of a VBC system for the treatment of fecal incontinence in women (LIFE Study, NCT01655498)