Figures & data
Figure 1 HEMOPATCH (Sealing Hemostat) is a next-generation hemostatic pad.
Abbreviation: NHS-PEG, N-hydroxysuccinimide functionalized polyethylene glycol.
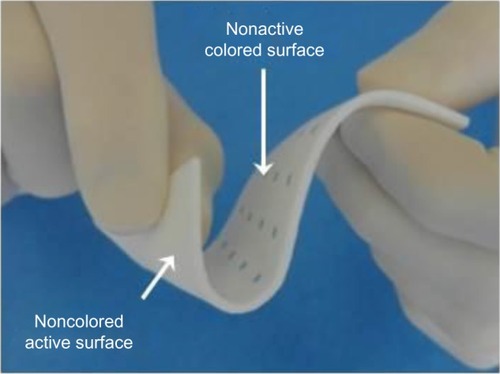
Figure 2 HEMOPATCH (Sealing Hemostat) binds to tissue through covalent amide bonds between PEG and tissue proteins and collagen.
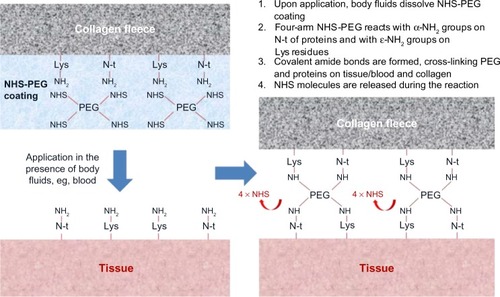
Figure 3 HEMOPATCH (Sealing Hemostat) collagen has an open pore structure that absorbs excess tissue fluid and delivers NHS-PEG to the tissue surface.
Abbreviations: NHS-PEG, N-hydroxylsuccinimide functionalized polyethylene glycol; SEM, scanning electron microscope.
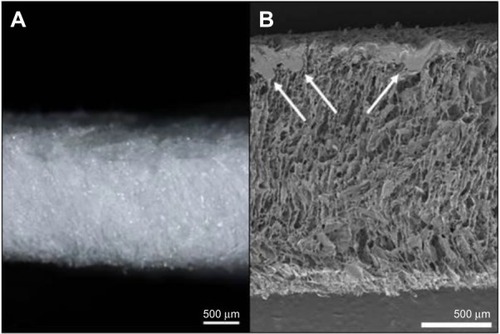
Figure 4 HEMOPATCH (Sealing Hemostat) is applied to a partial nephrectomy in a swine.
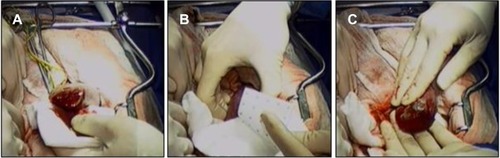
Figure 5 HEMOPATCH provided 100% hemostasis immediately after application, while TACHOSIL provided 80% hemostasis.
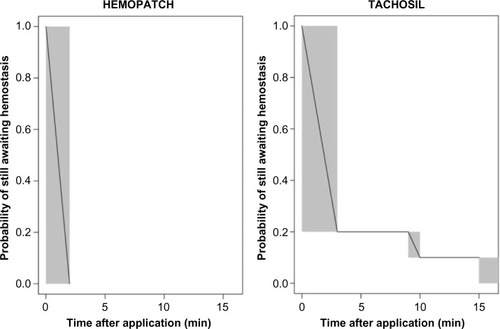
Figure 6 HEMOPATCH provides superior hemostatic performance and significantly less blood loss than TACHOSIL.
Abbreviation: CI, confidence interval.
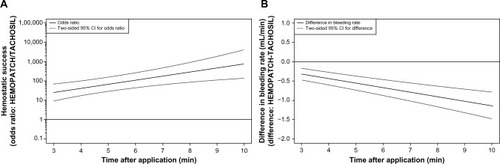
Table 1 HEMOPATCH (Sealing Hemostat) can withstand normal pressures of fluid-filled cavities when applied in the presence and absence of blood on a tissue substrate
