Figures & data
Table 1 MMPs upregulated in cardiovascular pathologies compared to relevant normal tissues, and cellular sources
Figure 1 Domain structure for the major classes of matrix metalloproteinases (MMPs). Major domains include the signal peptide (SP), prodomain (Pro), catalytic domain with the active site zinc bound to cysteine residues within this domain and “cysteine-switch” residue in the Pro, the hinge domain (HG), the hemopexin domain, and in some cases either a transmembrane domain (TM) or glycosylphosphatidylinositol (GPI)-anchor domain. A furin-cleavage site (F) between the Pro and the catalytic domain is found in some MMPs. In the gelatinases, fibronectin (FN)-like type II repeats are also present. The schematic within the dotted lines depicts the inhibitory action of tissue inhibitors of metalloproteinases (TIMPs) on MMPs, demonstrating how the N-terminus of the TIMP chelates the active site zinc and blocks MMP activity.
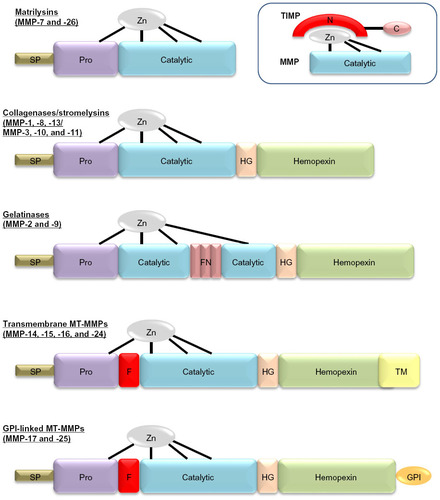
Table 2 Results of in vivo animal studies evaluating the effects of modulating matrix metalloproteinases (MMPs) or tissue inhibitors of metalloproteinases (TIMPs) on neointimal and vascular smooth-muscle cell behavior, using recombinant adenoviral overexpression, gene knockout, or pharmacological inhibitors of MMPs (MMPis)
Table 3 Results of in vivo animal studies evaluating the effects of modulating matrix metalloproteinases (MMP) or tissue inhibitors of metalloproteinases (TIMPs) on atherosclerotic plaque size and cellular composition, using transgenic (Tg) or recombinant adenoviral (RAd) overexpression, gene knockout (KO), or pharmacological inhibitors of MMPs
Table 4 Results of in vivo animal studies evaluating the effects of modulating matrix metalloproteinases (MMPs) or tissue inhibitors of metalloproteinases (TIMPs) on abdominal aortic aneurysm formation and cellular composition, using recombinant adenoviral (RAd) overexpression, gene knockout (KO), or pharmacological inhibitors of MMPs (MMPis)
Table 5 Results of in vivo animal studies evaluating the effects of modulating matrix metalloproteinases (MMPs) or tissue inhibitors of metalloproteinases (TIMPs) on post-MI remodeling, using recombinant adenoviral (RAd) overexpression (Ovex), gene knockout (KO [KO* < heterozygote]), gene knockdown (KD), transgenic (Tg), or pharmacological inhibitors of MMPs (Inhib)
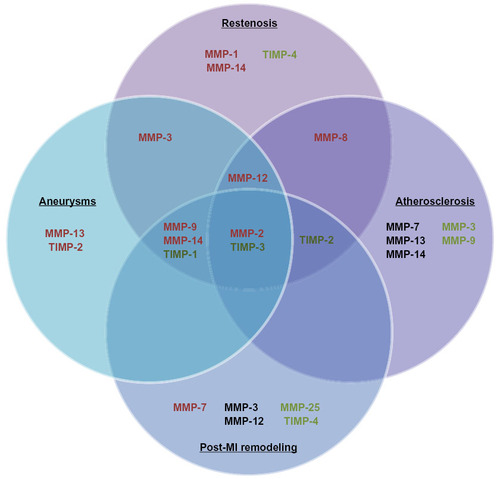
Figure 2 This diagram illustrates the matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) determined detrimental (red), neutral (black), or beneficial (green) in animal models of intimal formation, abdominal aortic aneurysms, atherosclerosis, and post-myocardial infarction (MI) remodeling.