Figures & data
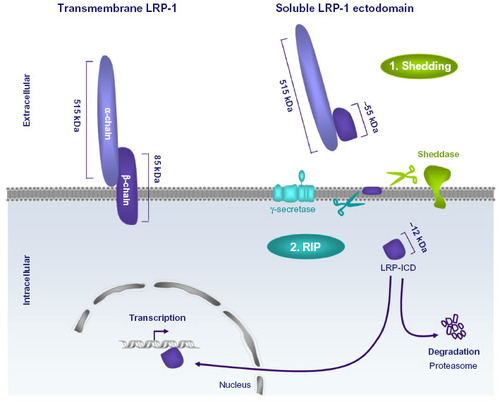
Figure 1 The low-density lipoprotein (LDL) receptor gene family.
Abbreviation: VLDL, very low-density lipoprotein.
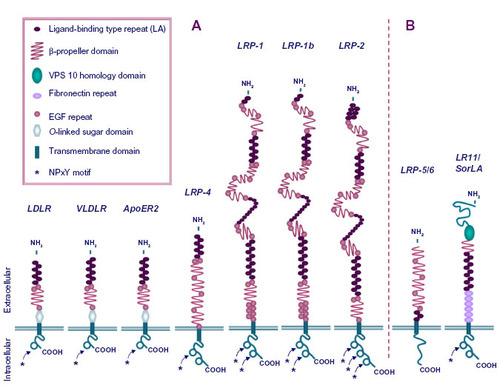
Figure 2 Different mechanisms of low-density lipoprotein receptor-related protein 1 (LRP-1)-mediated clearance of matrix metalloproteinases (MMPs).
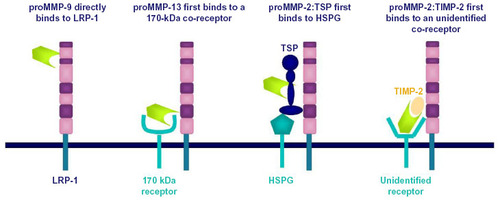
Figure 3 Low-density receptor-related protein 1 (LRP-1) ectodomain shedding and regulated intramembrane proteolysis.