Figures & data
Table 1 Demographics for Study I (open-label 4-week study consuming 1 g ACE daily)
Figure 1 Study design for the open-label pilot study on the aqueous cyanophyta extract (ACE) from Arthrospira platensis.
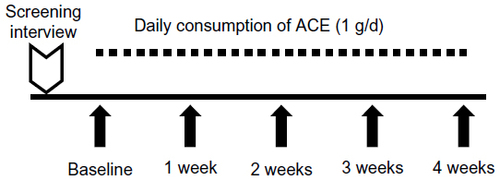
Table 2 Demographics for Study II (placebo-controlled crossover dose study)Table Footnotea
Figure 2 Study design for the single-blind, placebo-controlled dose study.
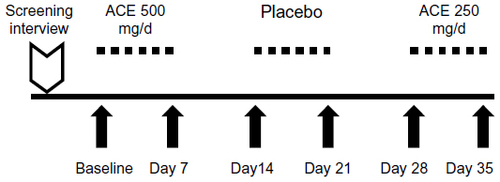
Figure 3 Data on pain scores collected by visual analog scales (VAS) during Study I.
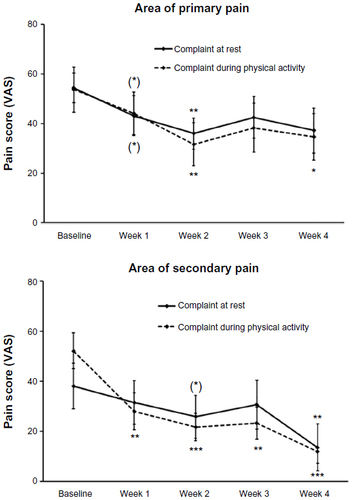
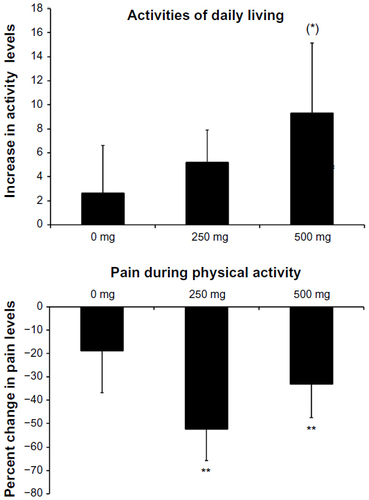
Figure 4 Data on activities of daily living questionnaire and pain level during physical activity were collected during Study II.
Abbreviation: ACE, aqueous cyanophyta extract.