Figures & data
Figure 1 Research flow chart.
Abbreviations: AIMS, abnormal involuntary movement scale; ALT, alanine transaminase; AST, aspartate aminotransferase; BARS, Barnes akathisia scale; CGI-S, clinical global impression-severity; ER, extended-release; MATRICS, measurement and treatment research to improve cognition in schizophrenia; MCCB, MATRICS consensus cognitive battery; PANSS, positive and negative syndrome scale; PSP, personal and social performance; SAS, Simpson–Angus scale.
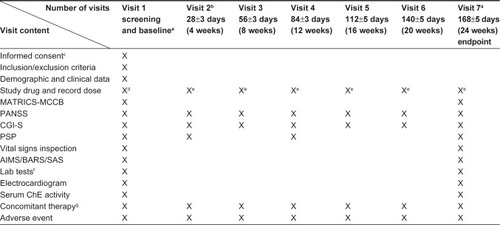
Table 1 Demographic and baseline data of full analysis set (FAS, n=90)
Table 2 Prestudy medications
Figure 2 Social function improvement after 24 weeks of treatment with paliperidone.
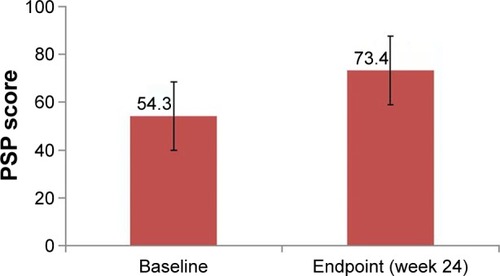
Table 3 Proportion of participants exhibiting changes in PSP scores in each dimension at each visit
Figure 3 Neurocognitive improvements after 24 weeks of treatment with paliperidone (t score).
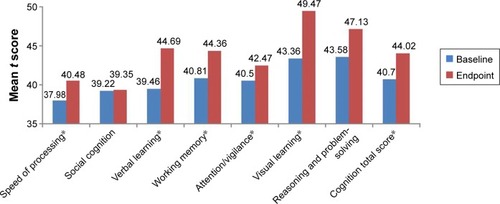
Table 4 Improvements in neurocognitive test scores after 24 weeks of treatment with paliperidone (raw scores)
Figure 4 Changes in PANSS total score after treatment with paliperidone.
Abbreviations: PANSS, positive and negative syndrome scale; SD, standard deviation.
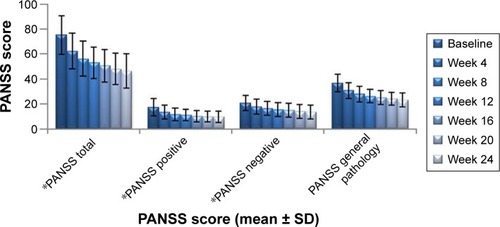
Figure 5 Changes in CGI-S score after treatment with paliperidone.
Abbreviations: CGI-S, clinical global impression-severity; SD, standard deviation.
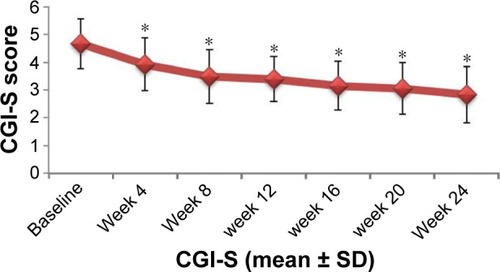
Table 5 Treatment-emergent adverse events
