Figures & data
Table 1 Baseline sociodemographic and clinical characteristics of CO-MED trial participants according to baseline activity impairment
Figure 1 Impairment in ability to perform routine day-to-day activities during acute phase of CO-MED trial (NCT00590863).
Abbreviation: CO-MED, Combining Medications to Enhance Depression Outcomes.
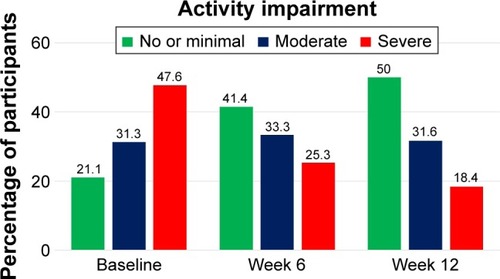
Figure 2 Change in activity impairment during acute phase of CO-MED trial (NCT00590863) based on treatment arm.
Abbreviations: CO-MED, Combining Medications to Enhance Depression Outcomes; SSRI, selective serotonin reuptake inhibitor.
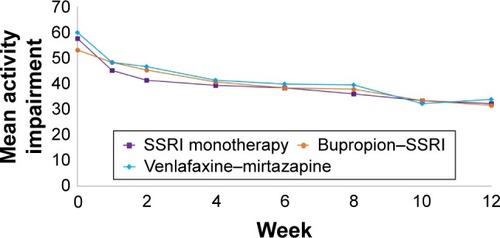
Table 2 Correlation coefficients of activity impairment and self-reported depression symptoms, functional outcomes, and side effects
Figure 3 Depression severity levels of depressed outpatients (n=665) in the CO-MED trial (NCT00590863) based on week 6 activity impairment category.
Abbreviations: CO-MED, Combining Medications to Enhance Depression Outcomes; QIDS-SR, Quick Inventory of Depressive Symptomatology, self-report.
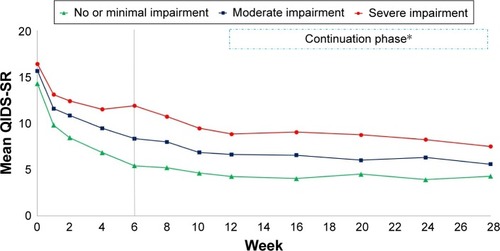
Table 3 Prediction of short- and long-term remission based on activity impairment categories at week 6
