Figures & data
Table 1 Patient background and baseline characteristics
Table 2A Time course changes in the number of medications concomitant with lamotrigine
Table 2B Time course changes in the mean dose of medications concomitant with lamotrigine
Table 3 Clinical Global Impressions Improvement scale scores at week 52/withdrawal
Figure 1 Time course changes in adherence to lamotrigine treatment.
Abbreviation: NOS, not otherwise specified.
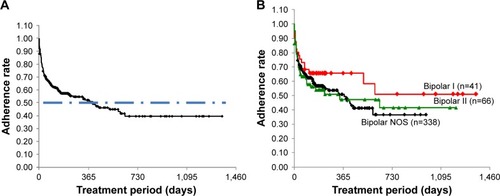
Table 4 Time course changes in HSDS and HSAS scores with and without antidepressants
Table 5 Adverse events experienced by the patients
