Figures & data
Figure 1 Information components and data communication.
Abbreviations: HCP, health care provider; IEM, ingestible event marker; MDDS, Medical Device Data System.
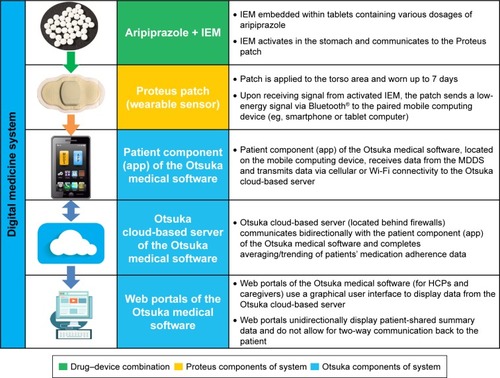
Figure 2 Steps in (A) human factors studies overall and (B) in risk analysis.
Abbreviations: ALARP, as low as reasonably practical; DMS, digital medicine system; FDA, US Food and Drug Administration; HCP, health care provider; NAC, not acceptable; RAC, risk analysis code; TOL, tolerable; uFMEA, use failure mode and effect analysis.
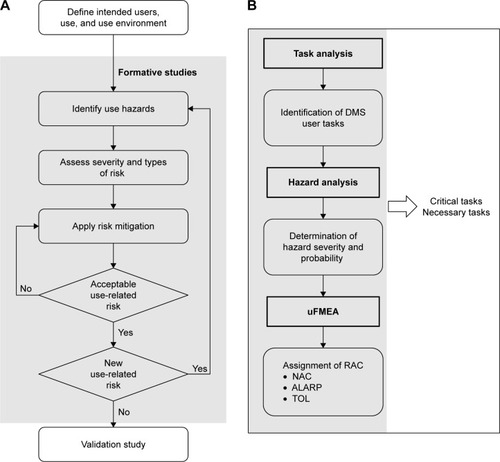
Table 1 Overview of human factors studies
Table 2 Examples of formative study findings and corresponding design modifications to the patient interface
Table 3 Performance assessment for tasks in the validation study of patient interface
Figure 3 Reduction in the percentage of failures on performance tasks and knowledge assessments during human factors testing of the patient interface.
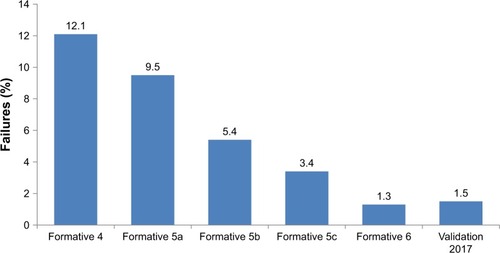
Table S1 Examples of user tasks tested in formative studies of patient interface
Table S2 Cautionary statements with a potential for serious or higher severity harm evaluated in the validation study
Table S3 App messaging evaluated in the validation study
