Figures & data
Figure 1 Study design.
Abbreviation: RAIM, rapid-acting intramuscular olanzapine.
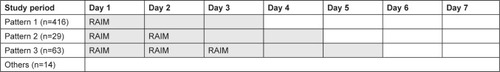
Table 1 Baseline patient characteristics and concomitant drug therapies
Table 2 Oral olanzapine dose after administration of 10 mg on the last day of rapid-acting intramuscular olanzapine
Figure 2 PANSS-EC score.
Abbreviations: d, days; h, hours; LOCF, last observation carried forward; n, number of patients; PANSS-EC, Positive and Negative Syndrome Scale-Excited Component; PO, Per Os (by mouth, orally); RAIM, rapid-acting intramuscular olanzapine.
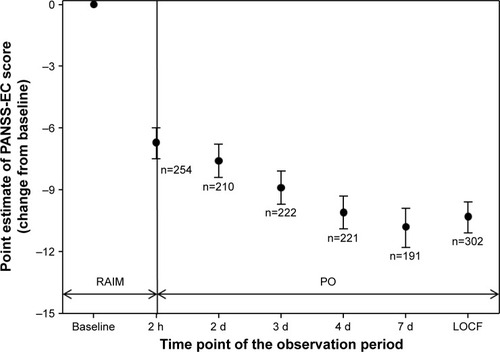
Table 3 TEAEs and adverse drug reactions based on the dose pattern of olanzapine
Table S1 Oral olanzapine dose after administration of 5 and 20 mg on the last day of rapid-acting intramuscular olanzapine
