Figures & data
Figure 1 Plasma concentration at trough (mean ± SE)–time profile during and following 12-weeks of treatment with cariprazine 6 mg/day.
Abbreviations: CAR, cariprazine; DDCAR, didesmethyl-cariprazine; total CAR, summed concentration of cariprazine, DCAR, and DDCAR.
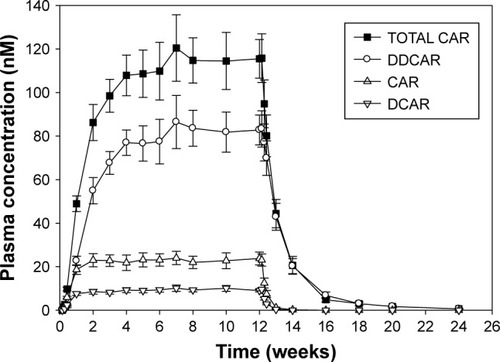
Table 1 Completed, randomized, placebo-controlled, phase II/III double-blind clinical trials of cariprazine for acute schizophrenia
Table 2 Potentially relevant adverse events associated with the use of cariprazine
Table 3 Potentially relevant adverse events associated with the use of cariprazine 1.5 mg/day as observed in Durgam et alCitation15,Table Footnotea
Figure 2 Kaplan–Meier curves of cumulative rate of relapse during the double-blind treatment period.
Abbreviations: DB, double-blind; DDCAR, didesmethyl-cariprazine.
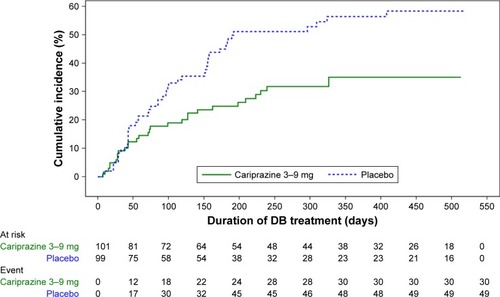
Table 4 Completed, longer term, randomized, controlled, phase III double-blind clinical trials of cariprazine for schizophrenia
Table 5 NNH vs placebo for approved first-line oral second-generation antipsychotics in adults, as observed in acute short-term studies for schizophreniaTable Footnotea
