Figures & data
Table 1 Patient demographics at the Royal Institute of Mental Health Research in a double-blind randomized trial of antidepressant monotherapy vs combination treatment, using escitalopram and bupropion in major depressive disorder patients
Figure 1 Patient enrollment at the Royal Institute of Mental Health Research in a double-blind, randomized trial of antidepressant monotherapy vs combination treatment, using escitalopram and bupropion in major depressive disorder patients.
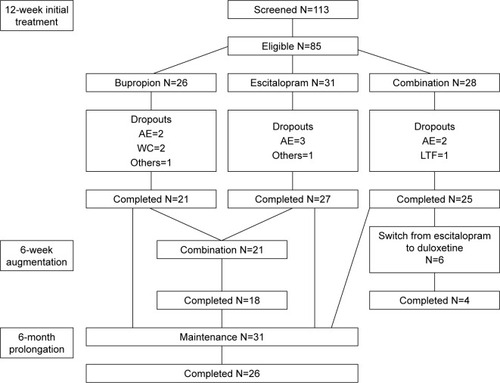
Figure 2 Mean scores on the HAM-D17 (0–42) by week, for all major depressive disorder patients (last observation carried forward) during 12 weeks of initial treatment at the Royal Institute of Mental Health Research in a double-blind, randomized trial of antidepressant monotherapy vs combination treatment, using escitalopram and bupropion.
Abbreviations: HAM-D 17, 17-item Hamilton Rating Scale for Depression; LSD, least significant difference.
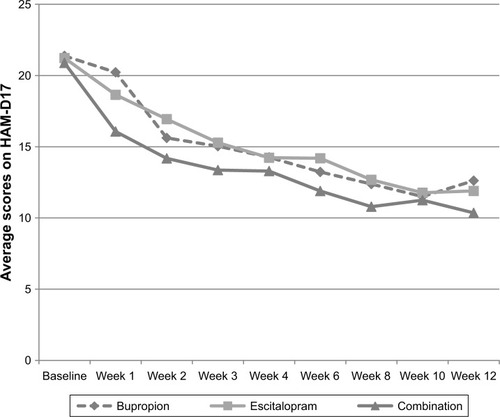
Figure 3 Percentage of remission on the HAM-D17 (≤7) by week, for all major depressive disorder patients (last observation carried forward) during 12 weeks of initial treatment at the Royal Institute of Mental Health Research in a double-blind randomized trial of antidepressant monotherapy vs combination treatment, using escitalopram and bupropion.
Abbreviation: HAM-D 17, 17-item Hamilton Rating Scale for Depression.
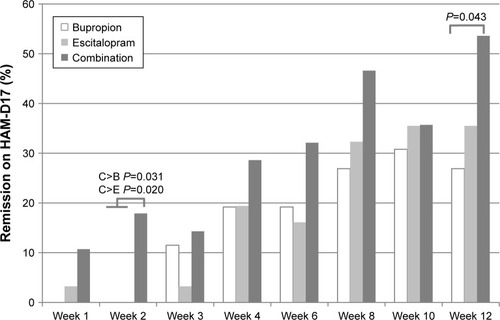
Figure 4 Percentage of remission on the MADRS (≤10) by week, for all major depressive disorder patients (last observation carried forward) during 12 weeks of initial treatment at the Royal Institute of Mental Health Research in a double-blind randomized trial of antidepressant monotherapy vs combination treatment, using escitalopram and bupropion.
Abbreviation: MADRS, Montgomery–Asberg Depression Rating Scale.
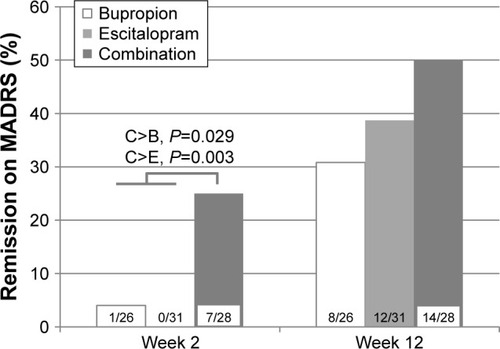
Table 2 Mean scores on the HAM-D17 (0–42) in a double-blind, 6-week augmentation trial in escitalopram or bupropion monotherapy non-remitters who received augmentation with the other drug, and in escitalopram and bupropion combination non-remitters who switched escitalopram for duloxetine (last observation carried forward)
Figure 5 Mean scores on the HAM-D17 (0–42) in a double-blind, 6-week augmentation trial in escitalopram or bupropion monotherapy non-remitters who received augmentation with the other drug, and in escitalopram and bupropion combination non-remitters who switched escitalopram for duloxetine (last observation carried forward).
Abbreviation: HAM-D 17, 17-item Hamilton Rating Scale for Depression.
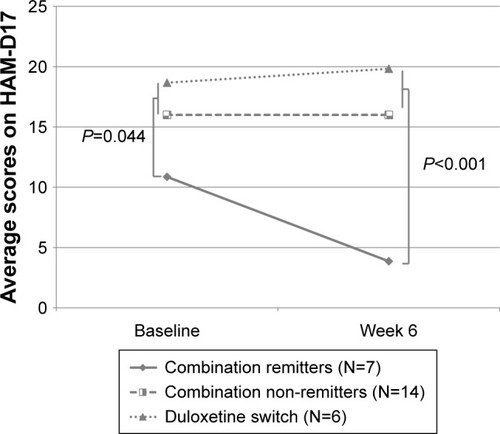
Figure 6 Mean scores on the HAM-D17 (0–42) in a double-blind 6-month prolongation in week 12 initial treatment and week 6 augmentation remitters (last observation carried forward) on antidepressant monotherapy or combination treatment, using escitalopram and bupropion.
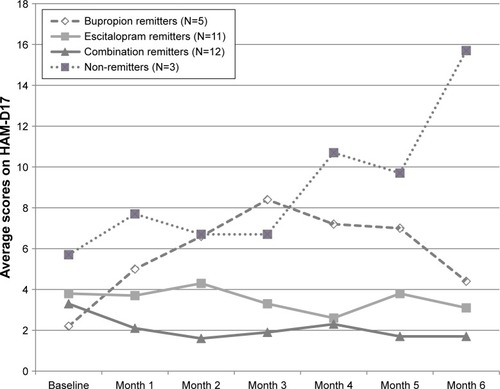
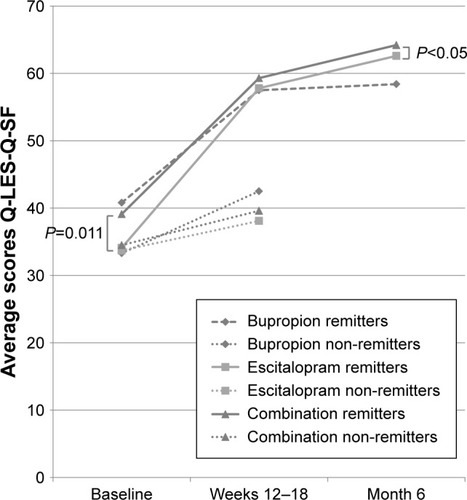
Figure 7 Mean scores on the Q-LES-Q-SF (0–80) for all patients (last observation carried forward) enrolled in a randomized, double-blind 12-week trial of antidepressant monotherapy vs combination treatment, including a double-blind 6-week augmentation in non-remitters, and a double-blind 6-month prolongation in both week 12 and week 18 remitters.