Figures & data
Table 1 Overlap between anxious distress and anxious depression
Table 2 Patient disposition stratified by the presence or absence of anxious distress and anxious depression at baseline
Table 3 Baseline demographic and clinical characteristics stratified by the presence or absence of anxious distress and anxious depression at baseline
Figure 1 Effect of brexpiprazole and placebo as adjunct to ADT on MADRS Total score, stratified by the presence or absence of anxious distress (A, B) and anxious depression (C, D) at baseline.
Abbreviations: ADT, antidepressant treatment; LS, least squares; MADRS, Montgomery–Åsberg Depression Rating Scale; SE, standard error.
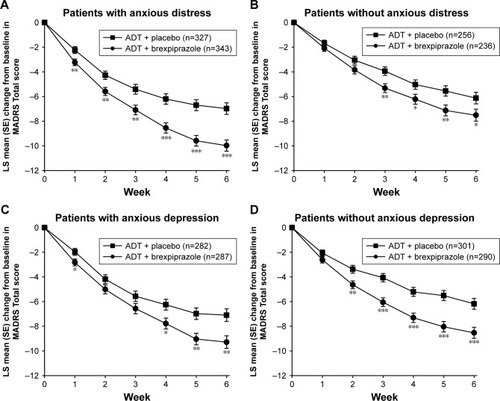
Table 4 TEAEs stratified by the presence or absence of anxious distress and anxious depression at baseline
