Figures & data
Figure 1 Study design and disposition of patients from Europe, Asia and rest of the world (ITT [OL] analysis set and mITT [DB] analysis set).
Abbreviations: DB, double-blind; EU, Europe; OL, open-label; PP1M, paliperidone palmitate 1-month; PP3M, paliperidone palmitate 3-month; ROW, rest of the world.
![Figure 1 Study design and disposition of patients from Europe, Asia and rest of the world (ITT [OL] analysis set and mITT [DB] analysis set).](/cms/asset/178f7ff9-a493-49f0-a807-7e61adb42ad2/dndt_a_189668_f0001_c.jpg)
Table 1 Analysis sets and number of patients by region/country in the study
Table 2 Demographic and baseline characteristics (ITT [OL] analysis set and mITT [DB] analysis set)
Table 3 Extent of exposure during the double-blind phase by region: Europe vs Asia vs rest of the world (safety analysis set)
Figure 2 Time-to-relapse during the double-blind phase and percentage of patients that remained relapse-free: (A) European patients; (B) Asian patients; (C) rest of the world patients (per-protocol analysis set).
Abbreviations: DB, double-blind; PP1M, paliperidone palmitate 1-month; PP3M, paliperidone palmitate 3-month.
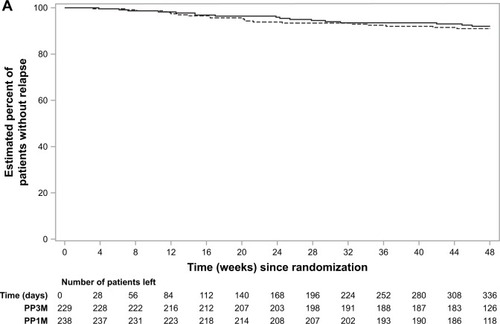
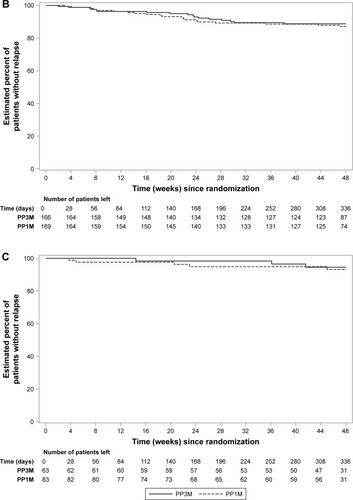
Figure 3 Mean (SE) total PANSS score (LOCF) over time: (A) European patients; (B) non-European patients (mITT [DB] analysis set).
![Figure 3 Mean (SE) total PANSS score (LOCF) over time: (A) European patients; (B) non-European patients (mITT [DB] analysis set).](/cms/asset/288a547a-09a2-4126-9f2f-c8804eb9c7a4/dndt_a_189668_f0003_c.jpg)
![Figure 3 Mean (SE) total PANSS score (LOCF) over time: (A) European patients; (B) non-European patients (mITT [DB] analysis set).](/cms/asset/c7055275-598b-4fb4-a4ff-27d161966f2c/dndt_a_189668_f0003a_c.jpg)
Figure 4 Percentage of European patients with (A) symptomatic remission; (B) symptomatic and functional remission over time during the double-blind phase (mITT [DB] analysis set).
![Figure 4 Percentage of European patients with (A) symptomatic remission; (B) symptomatic and functional remission over time during the double-blind phase (mITT [DB] analysis set).](/cms/asset/bb50a2ce-b4ab-412b-8234-12837ae6dd01/dndt_a_189668_f0004_c.jpg)
Table 4 Mean (SD) change from DB baseline in secondary efficacy parameters during the double-blind phase (mITT [DB] analysis set)
Table 5 Summary of treatment-emergent adverse events during the open-label (ITT [OL] analysis set) and double-blind phases (safety analysis set)
Table 6 Body weight, BMI, and waist circumference – change from baseline (OL) to endpoint (DB) – Europe vs non-Europe (Asia, rest of the world and total non-Europe) (safety analysis set)
