Figures & data
Figure 1 Patient disposition in groups 1 and 2.
Abbreviations: ADT, antidepressant therapy; BUP, buprenorphine; HAM-D, Hamilton Rating Scale for Depression; SAM, samidorphan.
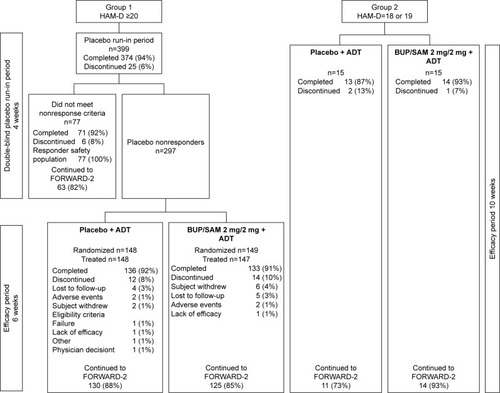
Table 1 Demographics and baseline clinical characteristics for randomized patients in the analysis population (group 1 placebo nonresponders)
Figure 2 Change from baseline in MADRS-10 total score over time in the analysis population.
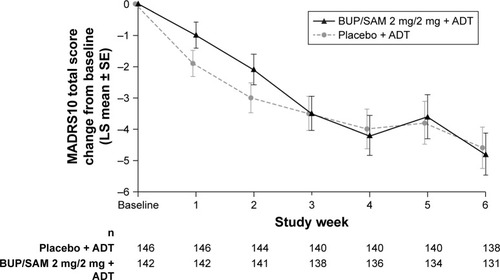
Table 2 Patients with treatment-emergent AEs during the 6-week postrandomization treatment period in the analysis population
Figure 3 MADRS-10 score change in the FORWARD-3 vs FORWARD-4 and FORWARD-5 studies during 6-week treatment period in stage 2.
Abbreviations: BUP, buprenorphine; LS, least squares; MADRS, Montgomery–Åsberg Depression Rating Scale; SAM, samidorphan.
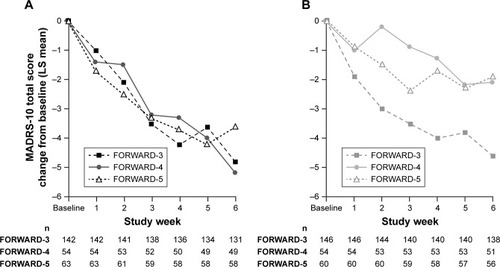
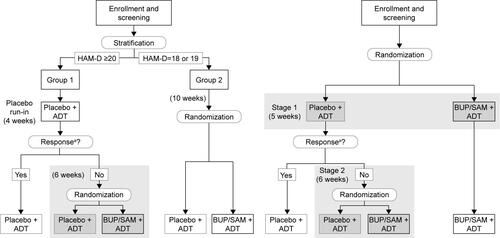
Figure S1 Comparison of trial designs in FORWARD-3 (left, double-blind run-in) vs FORWARD-4 and FORWARD-5 (right, sequential parallel-comparison design).
Notes: aResponse defined as ≥50% reduction in MADRS total score from baseline. Analysis populations indicated by dark gray tints; analysis stages indicated by light gray tint.
Abbreviations: ADT, antidepressant therapy; BUP, buprenorphine; HAM-D, 17-item Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale; SAM, samidorphan.
Availability of data and material
The data collected in this study are proprietary to Alkermes, Inc. Alkermes, Inc. is committed to public sharing of data in accordance with applicable regulations and laws.
