Figures & data
Figure 1 Endocannabinoid-mediated negative feedback in epilepsy: (1) in excitatory synapses, depolarization induces Glutamate (Glut) release into the synaptic cleft, thanks to the increase in intracellular Ca2+ levels mediated by the opening of voltage-gated calcium channels (VGCC); (2) in hyperexcitable states, like epileptic seizures, a large amount of neurotransmitter is released from the presynaptic neuron; (3) under basal conditions, Glut binds primarily to intra-synaptic ionotropic receptors (AMPARs); (4) in case of hyperexcitability with Glut “spill over”, group 1 metabotropic Glut receptors (ie, mGlu5Rs) located at pery-synpatic level are activated by ligand binding; (5) mGLU5Rs are anchored together with phospholipase C β (PLCβ) and diacylglycerol lipase α (DGLα) thanks to the scaffolding protein HOMER, forming a sopramolecular complex known as “2-AG signalosome” (illustrated in the smaller panel); (6) mGlu5R activation increases diacyl glycerol (DAG) synthesis by PLCβ and its following conversion into 2-arachydonoyl glycerol (2-AG), catalyzed by DGLα; (7) 2-AG acts as a retrograde messenger and binds pre-synaptic CB1 (coupled with Gi/o); (8) CB1 activation inhibits VGCC thus reducing intracellular Ca2+ levels; (9) ↓ Ca2+ levels determine a decrease in Glut exocytosis. This negative feedback mechanism could be protective against Glut-mediated excitotoxicity in hyperexcitable states.
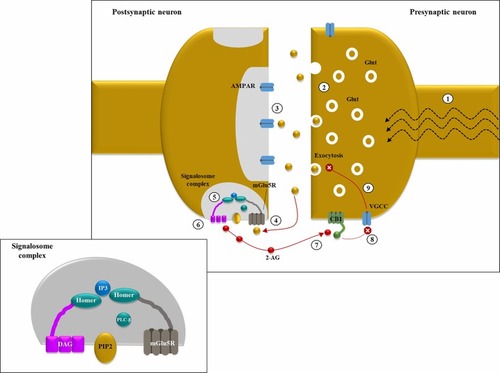
Table 1 Interactions Between CBD and Other AEDs
Figure 2 The figure shows the randomized, double-blind, placebo-controlled trials (followed by ongoing open-label extension studies) performed to evaluate CBD effectiveness and tolerability in DS, LGS and TSC. The percentages shown in the figure indicate the responder rates, ie, the proportion of patients showing >50% seizure reduction (for motor seizures in DS, drop seizures in LGS and convulsive ones in TSC) of CBD 20 mg/kg/day compared with placebo.
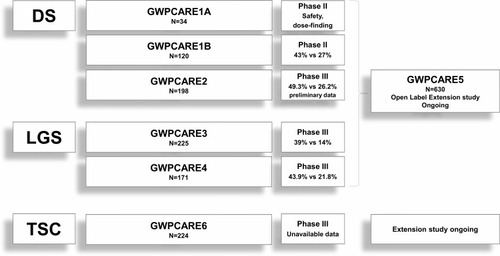
Table 2 AEs Reported in the Open-Label Extension Study (Interim Analysis)
