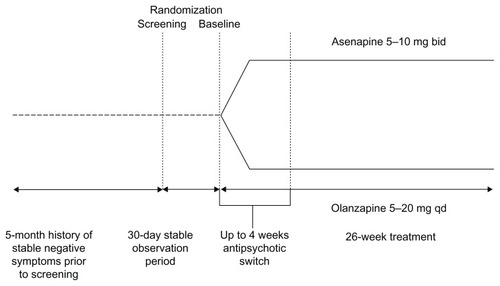
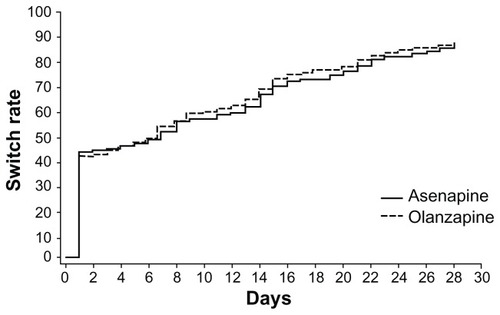
Figures & data
Table 1 Patient demographic and baseline characteristics
Table 2 Summary of pretreatment antipsychotic drug use during cross-titration period
Figure 3 Subjects reporting adverse events (AEs) by antipsychotic agent type during the switch period.
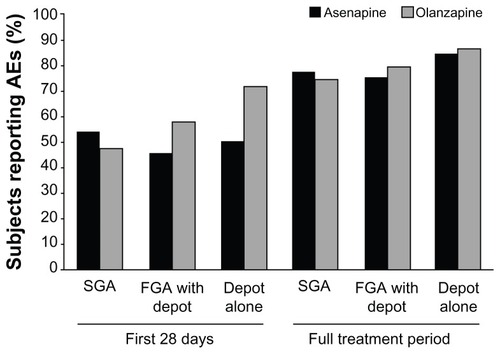
Table 3 Adverse events (AEs) in patients treated with second-generation antipsychotics during the switch period
Figure 4 Rate of (A) somnolence, (B) insomnia, and (C) incidence of weight gain by antipsychotic taken during the switch period.
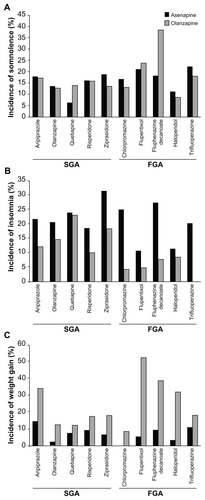
Table 4 Adverse events (AEs) in patients treated with first-generation antipsychotics (including depot) during the switch period
Table 5 Adverse events (AEs) in patients treated with depot medications during the switch period
Table 6 Effect of number of pre-switch antipsychotics used during switch on incidence of adverse events over the full treatment period (26 weeks)