Figures & data
Figure 1 Schedule for study treatments in (A) acute studies and (B) maintenance study.
Notes: (A) aEnrollment was a maximum of 28 days prior to randomization; bD1448C00009 and D1448C00011 only; cD1448C00009 and D1448C00010 only; down-titration in D1448C00009 only; dD1448C00010 only; eD1448C00011 only. (B) aTitration: Days 1–2 50 mg/day, Day 3 onwards 150 mg/day; btitration: Days 1–2 50 mg/day, Days 3–4 150 mg/day, Day 5 onwards 300 mg/day; cmaximum, or until anxiety event, or cessation of the study.
© Wolters Kluwer Health; reproduced with kind permission from Khan et alCitation21 and Katzman et alCitation25 respectively.
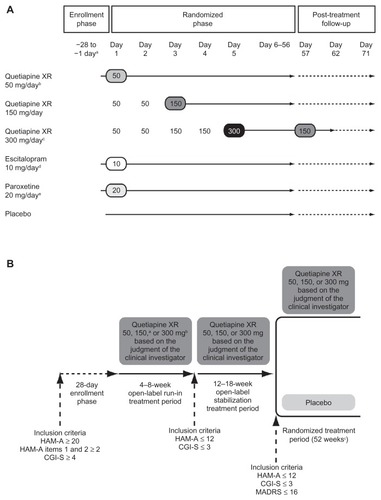
Table 1 Demographics and scores at randomization for HAM-A total and patient-reported outcome measures (pooled modified intent-to-treat population [acute studies] and intent-to-treat population [maintenance study])
Figure 2 Least squares mean change from randomization in Quality of Life and Satisfaction Questionnaire short form scores at (A) week 8 in pooled acute studies (modified intent-to-treat; last observation carried forward) and (B) during randomized treatment in the maintenance study (intent-to-treat; last observation carried forward).
Notes: (A) *P < 0.05; ***P < 0.001 vs placebo. n = placebo, quetiapine XR 50, 150 and 300 mg/day, respectively. (B) *P < 0.05; **P < 0.01; ***P < 0.001 vs placebo. n = placebo and quetiapine XR, respectively.
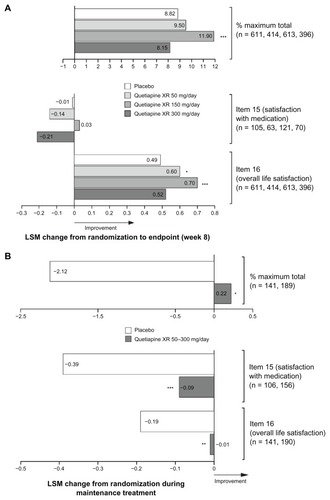
Figure 3 Least squares mean change from randomization in Pittsburgh Sleep Quality Index score at (A) week 8 in pooled acute studies (modified intentto- treat; last observation carried forward) and (B) during randomized treatment in the maintenance study (intent-to-treat; last observation carried forward).
Abbreviations: ITT, intent-to-treat; LSM, least squares mean; PSQI, Pittsburgh Sleep Quality Index; XR, extended release.
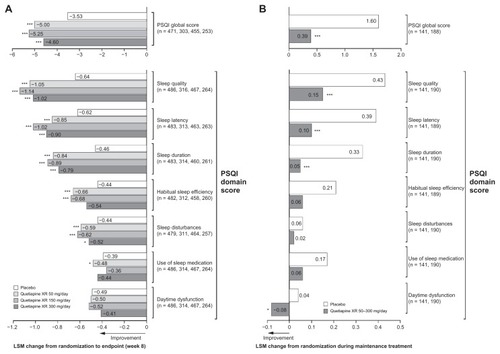
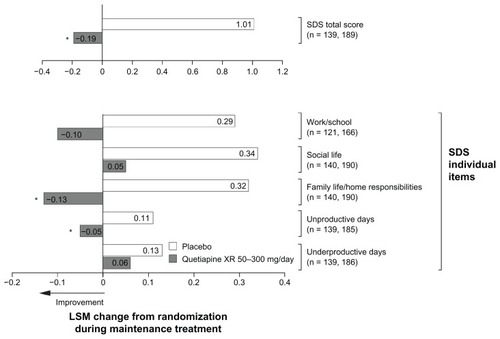
Figure 4 Least squares mean change from randomization in Sheehan Disability Scale score during randomized treatment in the maintenance study (intent-to-treat; last observation carried forward).
Notes: *P < 0.05 vs placebo. n = placebo and quetiapine XR, respectively.