Figures & data
Table 1 Patient Characteristics at Baseline in the Double Blind (DB) and Open-Label Extension (OLE) Phases
Figure 1 Mean (95% CI) change from baseline at each time point in Personal and Social Performance (PSP) total score during the double blind (DB) phase.
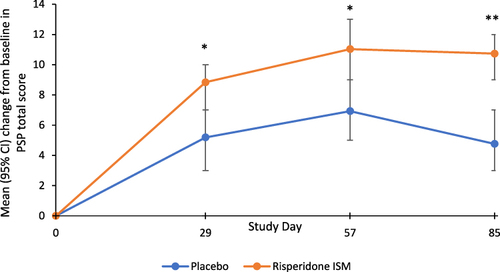
Figure 2 Mean domain scores of the Personal and Social Performance (PSP) scale at each assessment point during the double blind (DB) phase.
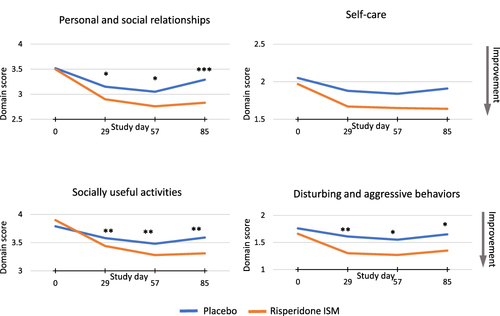
Figure 3 Mean change from baseline to each time point in Personal and Social Performance (PSP) scale total score during the open-label extension (OLE) phase.
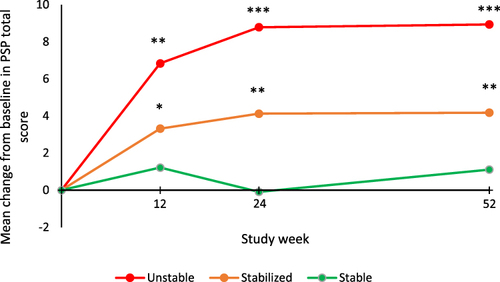
Figure 4 Mean domain scores of the Personal and Social Performance (PSP) scale at each assessment point during the open-label extension (OLE) phase.
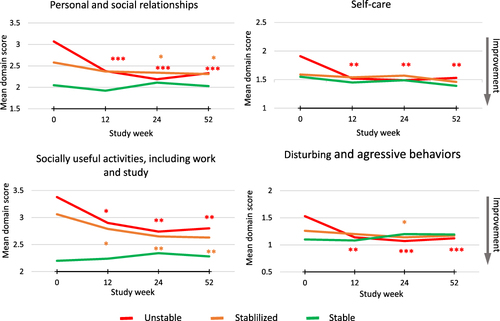
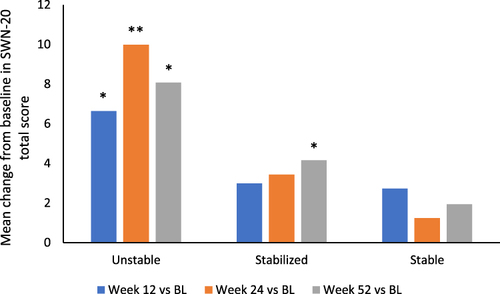
Figure 5 Mean change from baseline to each time point in SWN-20 total score during the open-label extension (OLE) phase.