Figures & data
Table 1 Summary of paliperidone palmitate initiation regimen simulations
Figure 1 Comparison of model-based projections versus observed data for the initial 5 weeks of treatment with PP 150 mg eq on day 1 followed by 100 mg eq on day 8. Abbreviations: mg eq, milligram equivalents; PP, paliperidone palmitate.
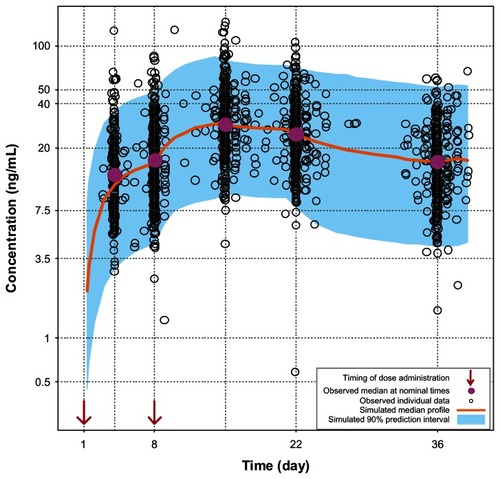
Figure 2 Comparison of model-based projections versus observed data for the initial 5 weeks of treatment with PP 150 mg eq on both days 1 and 8.
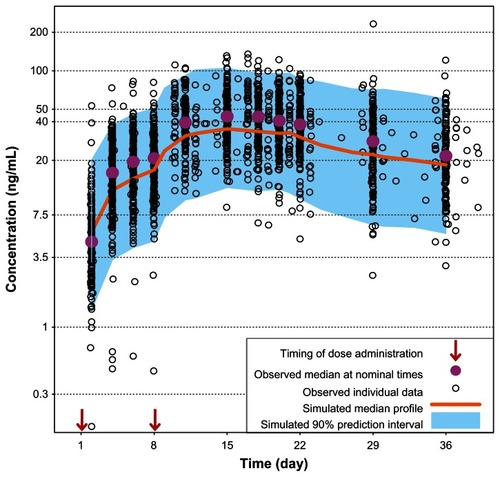
Figure 3 Comparison of model-based projections versus observed steady-state data for the recommended daily dose of paliperidone ER (6 mg).
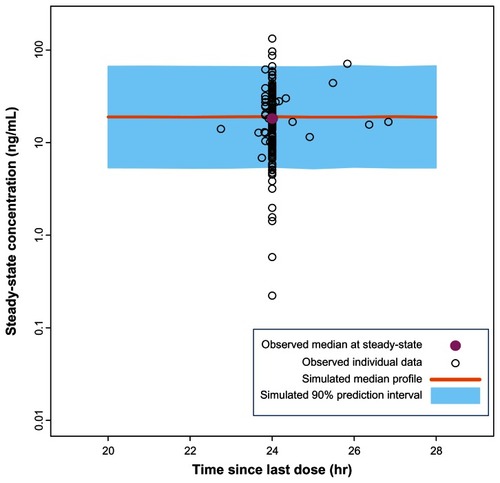
Figure 4 Pharmacokinetic simulations of the PP with second initiation dose (day 8) window expanded to ±4 days. (A) PP 150 mg eq on day 1 and 100 mg eq on day 8 versus 150 mg eq on day 1and 100 mg eq on day 4. (B) PP 150 mg eq on day 1 and 100 mg eq on day 8 versus 150 mg eq on day 1 and 100 mg eq on day 12.
Abbreviations: PP, paliperidone palmitate; mg eq, milligram equivalents.
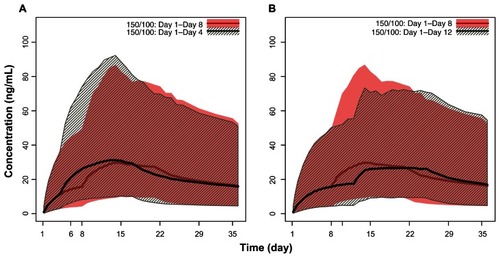
Table 2 Quantitative comparison of simulated peak exposures with paliperidone palmitate initiation regimens
Figure 5 Pharmacokinetic simulations for PP doses 150 mg eq and 100 mg eq with day 1/day 4, day 1/day 8, and day 1/day 12 initiation regimens compared with the highest initiation regimen (150 mg eq/day 1 followed by 150 mg eq/day 8). (A) PP 150 mg eq on both days 1 and 8 versus 150 mg eq on day 1 and 100 mg eq on day 4. (B) PP 150 mg eq on both days 1 and 8 versus 150 mg eq on day 1 and 100 mg eq on day 8. (C) PP 150 mg eq on both days 1 and 8 versus 150 mg eq on day 1 and 100 mg eq on day 12.
Abbreviations: PP, paliperidone palmitate; mg eq, milligram equivalents.
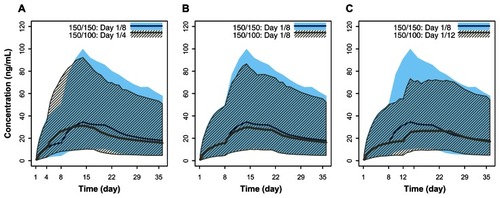
Figure 6 Pharmacokinetic simulations for PP doses 150 mg eq and 100 mg eq with day 1/day 4, day 1/day 8, and day 1/day 12 initiation regimens compared with the recommended daily dose of paliperidone ER (6 mg). (A) Steady-state 6 mg paliperidone ER versus PP 150 mg eq on day 1 and 100 mg eq on day 4. (B) Steady-state 6 mg paliperidone ER versus PP 150 mg eq on day 1 and 100 mg eq on day 8. (C) Steady-state 6 mg paliperidone ER versus PP 150 mg eq on day 1 and 100 mg eq on day 12.
Abbreviations: PP, paliperidone palmitate; ER, extended-release; mg eq, milligram equivalents.
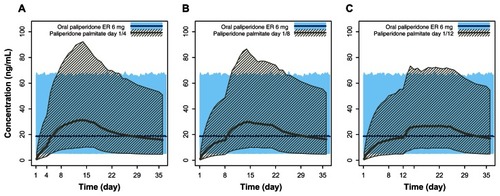
Table 3 Treatment-emergent adverse events reported in ≥5% of patients in any dose arm, by clinical trial