Figures & data
Figure 1 Patient disposition.
Abbreviations: MADRS, Montgomery–Åsberg Depression Rating Scale; MDD, major depressive disorder.
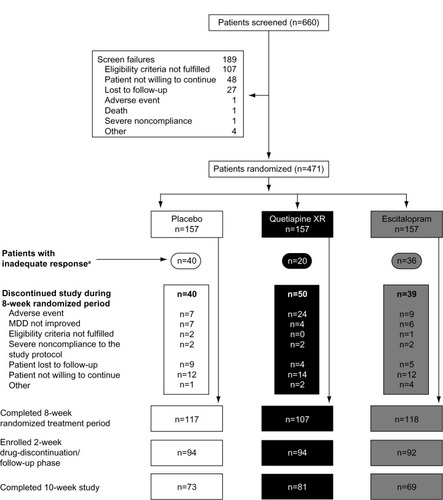
Table 1 Demographic and baseline clinical characteristics (MITT population)
Figure 2 (A and B) Change from randomization to week eight in MADRS total score. (A) LOCF approach; (B) MMRM analysis of observed-case data (MITT population).
Abbreviations: LOCF, last observation carried forward; LSM, least-squares mean; MADRS, Montgomery–Åsberg Depression Rating Scale; MITT, modified intent-to-treat; MMRM, mixed-model repeated measures.
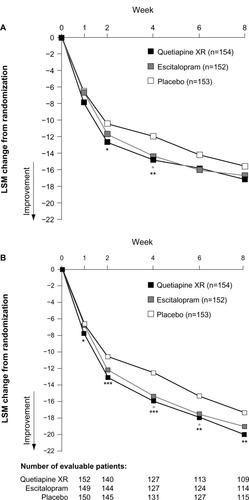
Figure 3 Change in MADRS total score over time in patients with an adequate and inadequate responsea,b at week two (LOCF; MITT population).
Abbreviations: LOCF, last observation carried forward; MADRS, Montgomery–Åsberg Depression Rating Scale; MITT, modified intent-to-treat.
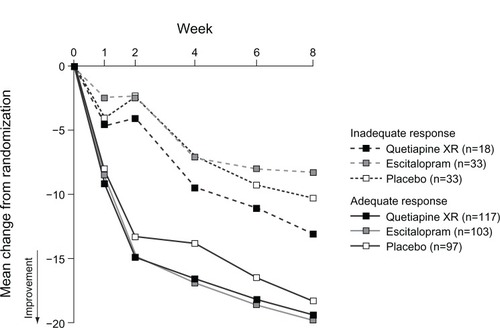
Figure 4 Change in individual MADRS item scores from randomization to week 8 (MITT population; LOCF).
Abbreviations: LOCF, last observation carried forward; LSM, least-squares means; MADRS, Montgomery–Åsberg Depression Rating Scale; MITT, modified intent-to-treat.
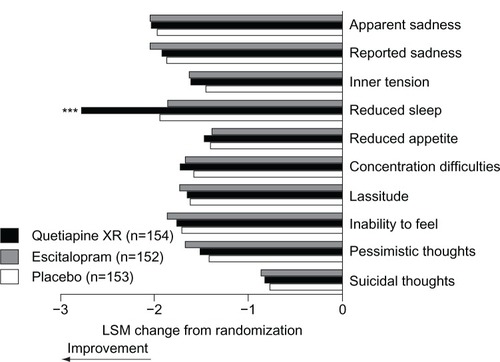
Table 2 Change from randomization at week one and at week eight in secondary efficacy variables (MITT population, LOCF unless otherwise stated) and TDSS total scores over time during the drug discontinuation/tapering follow-up phase (TDSS population; OC)
Table 3 LSM change in MADRS total score from randomization to week eight by age, sex, ethnicity, disease severity, and continent, and MADRS response rates by continent (LOCF; MITT population)Table Footnotea
Table 4 Most frequently reported AEs (occurring at an incidence of >5% in any group) and AEs of special interest (safety population)
Table 5 Changes in clinical laboratory parameters and body weight and proportion of patients with clinically relevant shifts (defined within the table) from randomization to end of treatment (safety population; LOCF)
