Figures & data
Figure 1 Patients’ treatments before and during the original studies, and patient groups in the post hoc analyses. Only patients who had used OLZ or non-OLZ immediately before randomization to OLAI were included in the post hoc analyses.
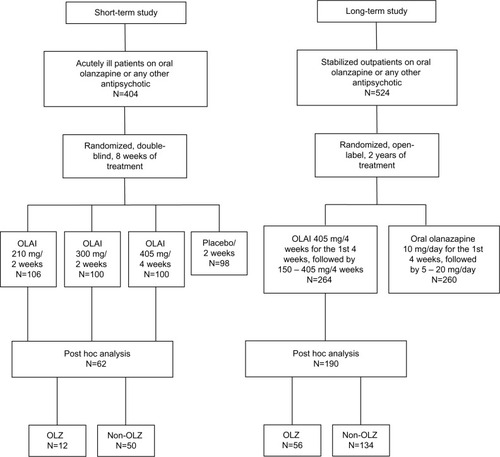
Table 1 Antipsychotic medications used immediately before starting the olanzapine long-acting injection
Table 2 Baseline patient demographics
Table 3 Baseline disease characteristics
Figure 2 Kaplan–Meier survival analysis of time to all-cause discontinuation from OLAI treatment. No statistically significant differences were detected in times to all-cause discontinuation in the short-term study or in the long-term study.
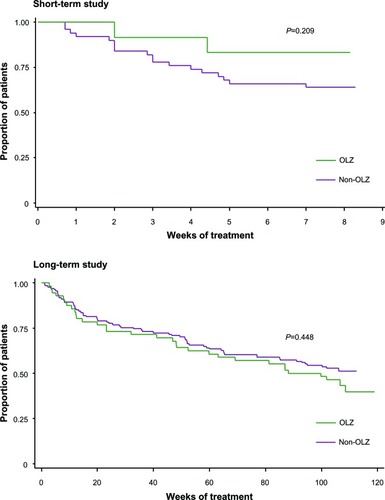
Figure 3 Rates of discontinuation and reasons for discontinuation from OLAI treatment. No statistically significant differences were detected in discontinuation rates in the short-term study or in the long-term study.
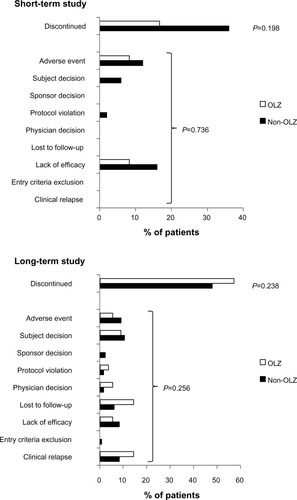
Table 4 Disease severity measures after treatment with olanzapine long-acting injection
Table 5 Treatment-emergent adverse events occurring in >5.0% and two or more patients
Table 6 Treatment-emergent serious adverse events occurring in two or more patients
Figure 4 Potentially clinically significant changes in metabolic parameters after switching to OLAI treatment. The only statistically significant difference detected in potentially clinically significant changes was “weight gain” in the short-term study. Definitions: weight gain, ≥7% increase from baseline; fasting glucose, ≥7 mmol/L following baseline of <5.56 mmol/L; fasting triglycerides, ≥2.26 mmol/L following baseline of <1.69 mmol/L; total fasting cholesterol, ≥6.21 mmol/L following baseline of <5.17 mmol/L; fasting LDL, ≥4.13 mmol/L following baseline <2.58 mmol/L; fasting HDL,<1.03 mmol/L following baseline of ≥1.03 mmol/L.
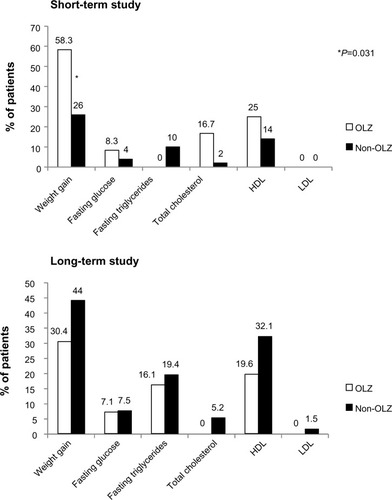
Figure 5 Potentially clinically significant changes in extrapyramidal symptoms at any time after switching to OLAI treatment. Statistically significant differences were detected in akathisia (in the short-term and long-term studies) and parkinsonism (in the long-term study).
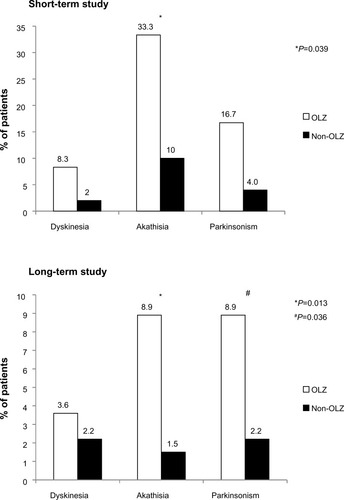
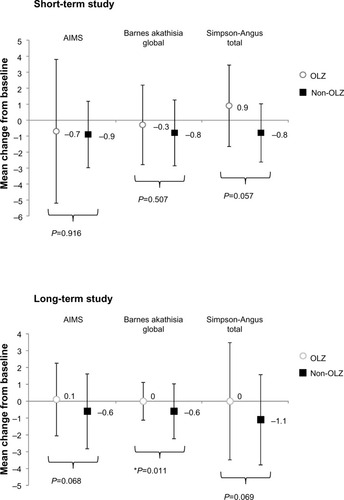
Figure 6 Mean (SD) changes in extra pyramidal symptom scores. The only statistically significant difference was detected in the Barnes Akathisia Rating Scale in the long-term study.