Figures & data
Figure 1 Patient disposition.
Abbreviation: Gal, galantamine.
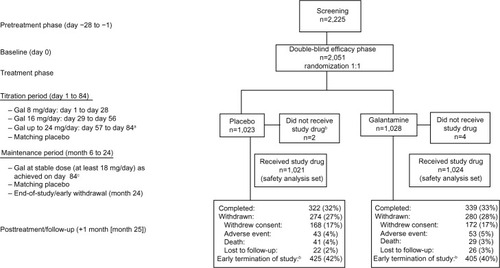
Table 1 Demographic and baseline characteristics (safety analysis set)
Table 2 Extent of exposure to galantamine and placebo
Table 3 Treatment-emergent adverse events in at least 2% of patients in any treatment group (safety analysis set)
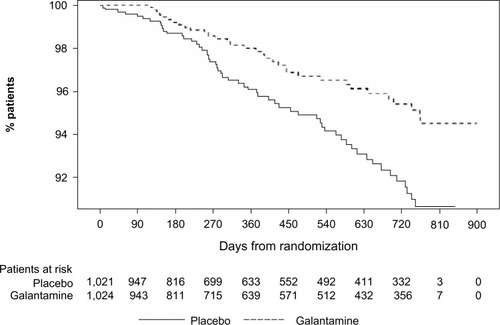
Figure 3 Mean change in MMSE scores over time (LOCF) (ITT analysis set).
Abbreviations: CI, confidence interval; GCP, Good Clinical Practice; ITT, intent-to-treat; LOCF, last observation carried forward; MMSE, Mini-Mental State Examination; OC, observed case; SE, standard error.
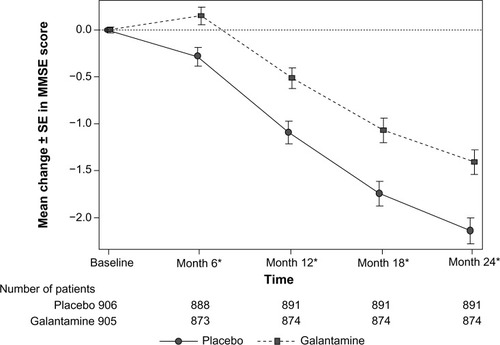
Figure 4 Mean change in DAD scores over time (LOCF) (ITT analysis set).
Abbreviations: DAD, Disability Assessment in Dementia; GCP, Good Clinical Practice; ITT, intent-to-treat; LOCF, last observation carried forward; SE, standard error.
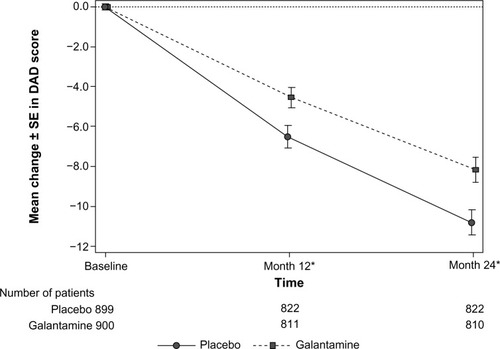
Table 4 Analysis of MMSE at month 24 by concomitant use or nonuse of memantine medication (intent-to-treat analysis set)
Table S1 Demographic and baseline characteristics (completers analysis set)
Table S2 Medical history (safety analysis set)
Table S3 Medical history (completer analysis set)