Figures & data
Table 1 Baseline demographics (safety population)Table Footnotea
Table 2 Summary of lifetime ADHD medications (safety population)
Figure 1 Change in ADHD-RS-IV total scores from baseline to endpoint in treatment-naïve and previously treated patients (full analysis set).
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ADHD-RS-IV, ADHD rating scale IV; CI, confidence interval; LDX, lisdexamfetamine dimesylate; LS, least-squares; MPH, methylphenidate; OROS-MPH, osmotic-release oral system MPH.
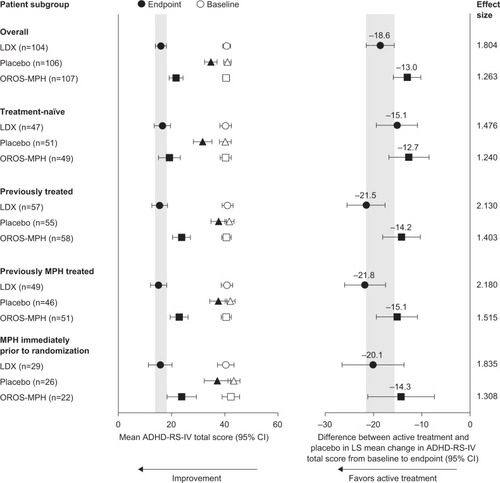
Table 3 Proportions of improved patients (CGI-I score of 1 or 2) at endpoint (full analysis set)
