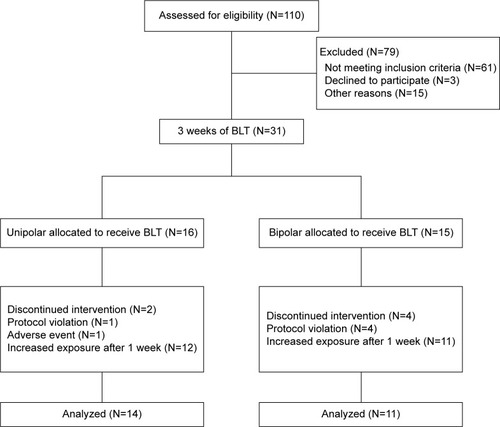
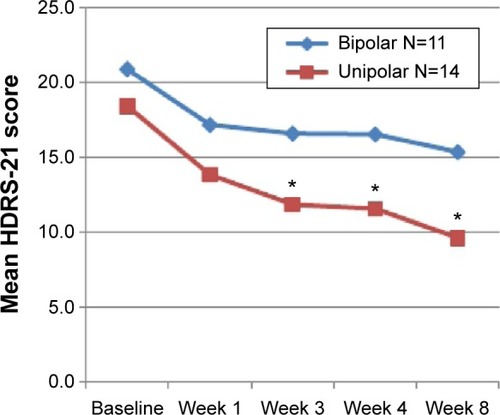
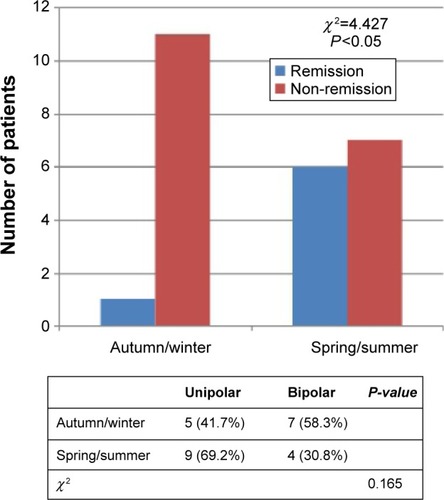
Figures & data
Table 1 Demographic and clinical features at baseline
Table 2 Psychometric features from all assessments, both for unipolar and bipolar patients
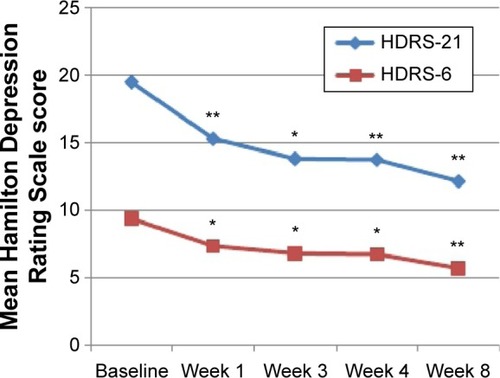
Figure 2 Mean overall HDRS-21 and HDRS-6 scores.
Notes: Repeated measures showed that mean HDRS scores significantly decreased since the first week of treatment. In comparison with baseline, a significant reduction was observed at week 1, week 3, week 4, and week 8. *P<0.05. **P<0.001.
Abbreviations: HDRS-21, Hamilton Depression Rating Scale (21 items); HDRS-6, Hamilton Depression Rating Scale (6 items).
Abbreviations: HDRS-21, Hamilton Depression Rating Scale (21 items); HDRS-6, Hamilton Depression Rating Scale (6 items).