Figures & data
Table 1 Cognitive and emotion tasks
Figure 1 Consort chart.
Abbreviations: QIDS-SR16, 16-item Quick Inventory of Depressive Symptomatology Self-Report; iSPOT-D, International Study to Predict Treatment Response in Depression; XR, extended release; HRSD17, 17-item Hamilton Rating Scale for Depression.
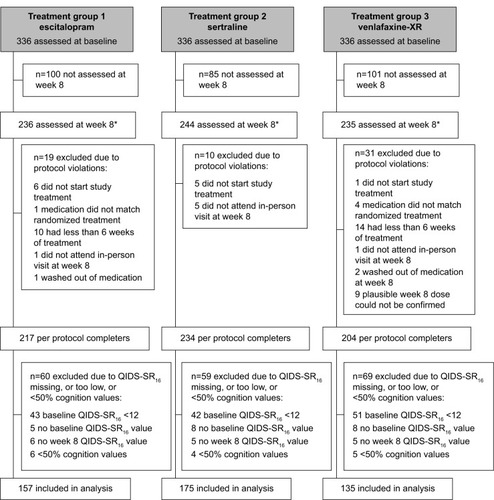
Table 2 Proportion of patients by treatment for whom a test battery recommendation could be made
Table 3 Sensitivity and specificity of the depression treatment test
Figure 2 Final regression model to predict remission in each treatment arm and applicable subgroup.
Abbreviations: DT, depression treatment test; QIDS-SR16, 16-item Quick Inventory of Depressive Symptomatology Self-Report; XR, extended release.
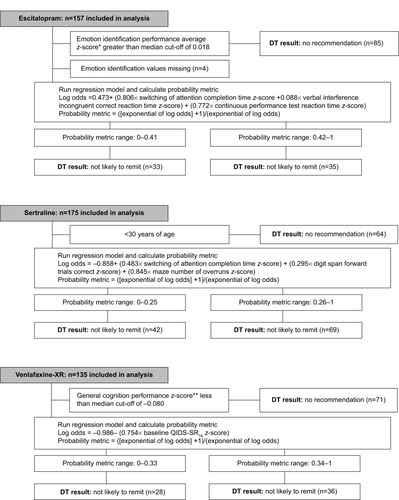
Figure 3 ROC curves comparing cognition with age and sex as well as depression severity.
Abbreviations: AUC, area under the curve; QIDS-SR16, 16-item Quick Inventory of Depressive Symptomatology Self-Report; ROC, receiver operating characteristics; XR, extended release.
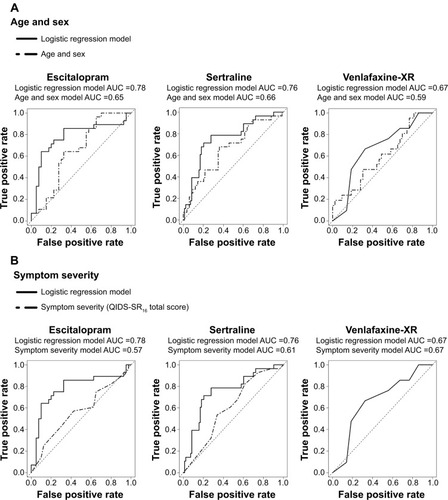
Table 4 Proportion of patients identified with greater than 80% certainty to remit
Table 5 Proportion of patients identified with greater than 80% certainty to not remit
Figure 4 Percent of the sample receiving a recommendation to remit or to not remit, and relative risks for each type of prediction.
Abbreviation: XR, extended release.
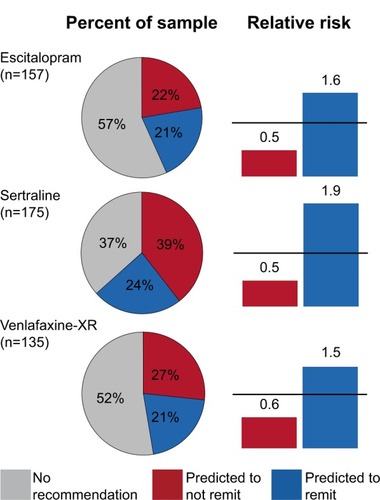
Table S1 Inclusion/exclusion criteria
Table S2 Comparison of included and excluded samples (categorical measures)
Table S3 Comparison of included and excluded samples (continuous measures)
