Figures & data
Table 1 Patient demographics and baseline characteristics
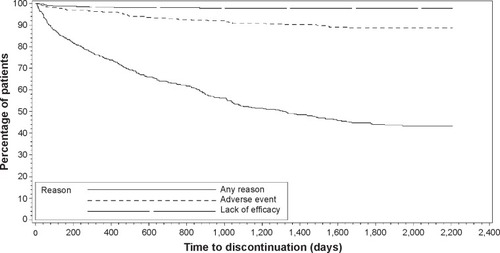
Table 2 Patient disposition
Figure 2 Positive and Negative Syndrome Scale total and subscale scores, change from baseline to endpoint (mixed model repeated measures analysis).
Abbreviations: N, number of patients; SE, standard error.
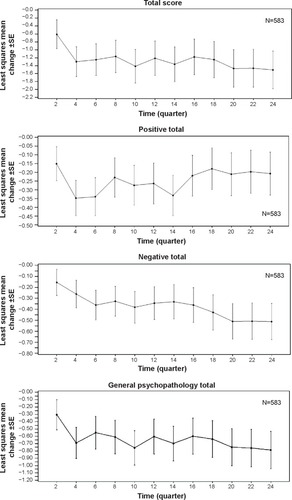
Figure 3 Maintenance of treatment effect. The CGI-S visit-wise mean score for all 669 patients (mean baseline =2.84, mean endpoint =2.62).
Abbreviation: CGI-S, Clinical Global Impression-Severity.
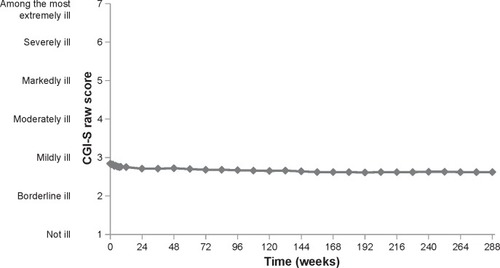
Figure 4 Categorical analysis of the Patient Satisfaction with Medication Questionnaire-Modified at endpoint (N=620).
Abbreviation: N, number of patients.
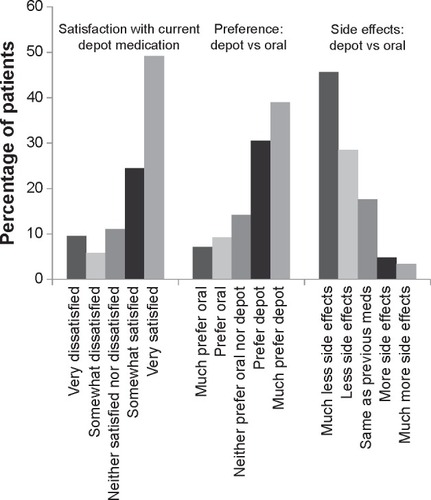
Table 3 Treatment-emergent adverse events in ≥5% of patients
Table 4 Mean changes in laboratory results and weight