Figures & data
Table 1 Demographic data of the generic escitalopram (Lexacure®) and branded escitalopram (Lexapro®) groups (ITT)
Figure 1 Changes in MADRS, HDRS, and CGI-S from baseline to week 6 (PP).
Abbreviations: CGI-S, Clinical Global Impressions-Severity scale; HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; PP, per protocol.
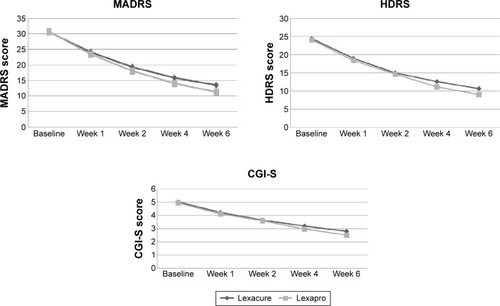
Table 2 Changes in MADRS scores and comparisons of the generic escitalopram (Lexacure®) and branded escitalopram (Lexapro®) groups at each study visit (PP)
Figure 2 Comparisons of response and remission rates between the generic escitalopram (Lexacure®) and branded escitalopram (Lexapro®) groups (PP).
Abbreviations: CGI-I, Clinical Global Impressions–Improvement scale; HDRS, Hamilton Depression Rating Scale; PP, per protocol; MADRS, Montgomery–Åsberg Depression Rating Scale.
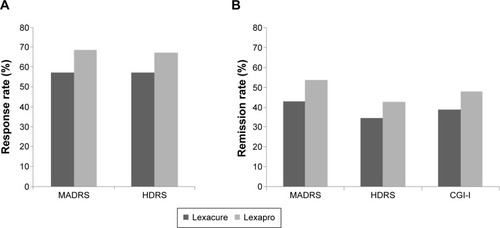
Table 3 The presence of adverse events in the generic escita-lopram (Lexacure®) and branded escitalopram (Lexapro®) groups (AEs >1.0%; ITT)
