Figures & data
Table 1 PCR primer sequence
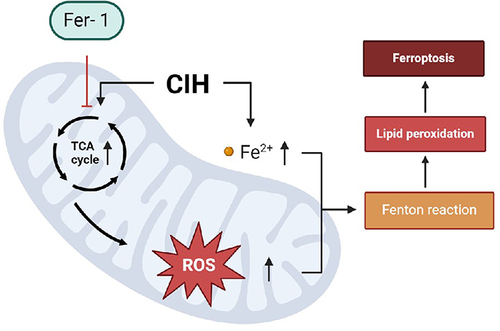
Figure 1 CIH-induced ROAEC injury and ferroptosis. (A), the apoptosis rate in the CIH group and control group; (B), the ROS levels in CIH group and control group; (C), the Fe2+ levels in CIH group and control group; (D), the lipid ROS levels in CIH group and control group; (E), the cell viability of ROAEC decreased in CIH group and control group; (F), The mRNA levels of GPX4 and SLC7A11 in CIH group and control group; (G), the protein levels of GPX4 and SLC7A11 in CIH group and control group; (H), the MDA levels in CIH group and control group; (I), the NAD+/NADH ratio in CIH group and control group. Data were repeated at least three times and shown as the mean ± SD. *p < 0.05; **p < 0. 01; ***p < 0. 001; ****p < 0. 0001.
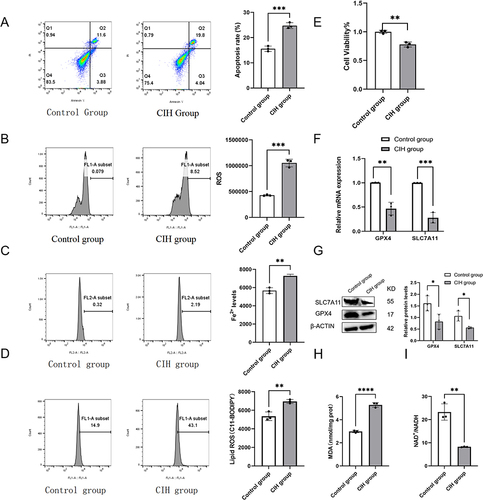
Figure 2 Fer-1 alleviated CIH-related ROAEC ferroptosis. The ROAEC were divided into control group, CIH group, and CIH+Fer-1 group. (A), co-treatment with Fer-1 reversed the CIH-induced apoptosis; (B), co-treatment with Fer-1 reversed the CIH-induced ROS accumulation; (C), co-treatment with Fer-1 reversed the increase of Fe2+ levels induced by CIH; (D), co-treatment with Fer-1 reversed the lipid ROS levels induced by CIH; (E), co-treatment with Fer-1 reversed the decline in cell viability induced by CIH; (F), the mRNA levels of SLC7A11 and GPX4 increased due to Fer-1 co-treatment; (G), the protein levels of SLC7A11 and GPX4 increased after Fer-1 co-treatment; (H), co-treatment with Fer-1 reversed the increase of MDA levels induced by CIH; (I), co-treatment with Fer-1 reversed the decline in NAD+/NADH ratio induced by CIH. Data are shown as the mean ± SD. Compared with the control group, *p < 0.05, **p < 0. 01, ***p < 0. 001, ****p < 0. 0001; Compared with the CIH group, # p < 0.05, ## p < 0. 01, ### p < 0. 001, ####p < 0. 0001.
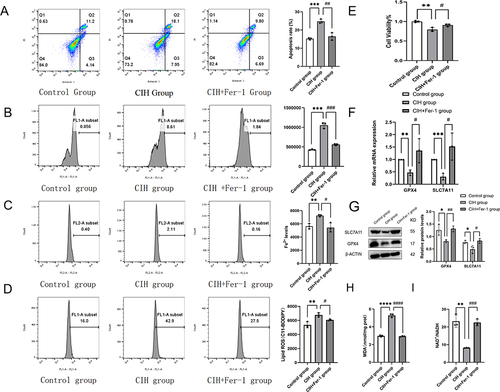
Figure 3 Fer-1 alleviated mitochondrial injury in CIH treated ROAEC. (A), Transmission electron microscopy (TEM) photo of the control group and CIH group; (B), Levels of mitochondrial membrane potential (MMP) in the control group and CIH group. (C), TEM photo of the control group, CIH group, and CIH+Fer-1 group. (D), Levels of MMP in the control group, CIH group, and CIH+Fer-1 group. Data are shown as the mean ± SD. Compared with the control group, **p < 0. 01; Compared with the CIH group, ####p < 0. 0001.
Figure 4 Fer-1 ameliorated CIH-induced metabolism abnormalities in the TCA cycle. Hierarchical clustering of significantly regulated metabolites of central carbon metabolism among the control group and CIH group (n = 6, biological replicates).(A) color scale indicates relative metabolite levels: red for up-regulated, blue for down-regulated. (B), Schematic of altered metabolites in TCA cycle.(C), Hierarchical clustering of significantly regulated metabolites of central carbon metabolism among the control group, CIH group, and CIH+Fer-1 group (n = 6, biological replicates). A color scale indicates relative metabolite levels: red for up-regulated, blue for down-regulated. (D), CIH increased the levels of metabolites in the TCA cycle. Fer-1 treatment alleviated the increase of metabolites induced by CIH. Data are shown as the mean ± SD. Compared with the control group, **p < 0. 01, ***p < 0. 001, ****p < 0. 0001; Compared with the CIH group, ### p < 0. 001, ####p < 0. 0001.
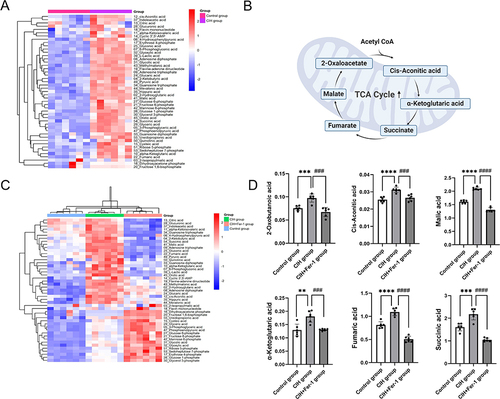
Figure 5 Possible molecular mechanisms of Fer-1 reversing CIH-induced ferroptosis in aortic endothelial cells. In ROAEC, after CIH treatment, the activity of important metabolites in the TCA cycle increased, which led to the accumulation of lipid ROS, the changes in mitochondrial membrane potential, and disruption of mitochondrial structure, eventually led to ferroptosis. Co-treatment with Fer-1 reversed ferroptosis via reprogramming the metabolites of the TCA cycle and mitochondrial function.