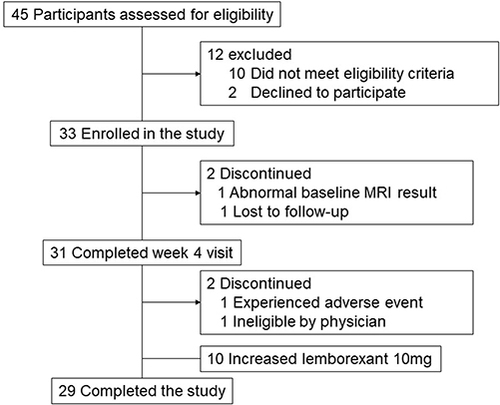
Figures & data
Figure 1 Test schedule. *The dose is increased to 10 mg (LEM10) after consulting with a study physician based on the participant’s complaints.
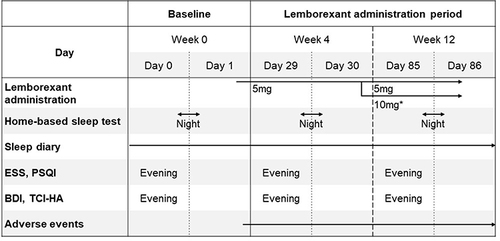
Table 1 Characteristics of Participants
Table 2 Objective Sleep Parameters at Baseline, and 4th and 12th Weeks
Table 3 Subjective Sleep Parameters at Baseline, and 4th and 12th Weeks
Table 4 Sleep Disturbance, Daytime Sleepiness, and Depressive Symptoms at Baseline, and 4th and 12th Weeks
Table 5 Safety Summary During Treatment and Follow-Up Periods