Figures & data
Figure 1 Visualization of SOD activity isoforms and determination of total SOD activity at various developmental stages of Helicoverpa armigera larvae.
Abbreviations: SOD, superoxide dismutase; NBT, nitroblue tetrazolium; ANOVA, analysis of variance; SD, standard deviation.
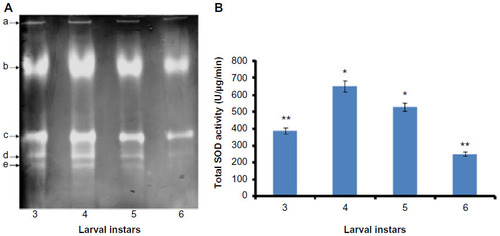
Figure 2 Effect of metal ions on Helicoverpa armigera total SOD activity.
Abbreviations: SOD, superoxide dismutase; SD, standard deviation.
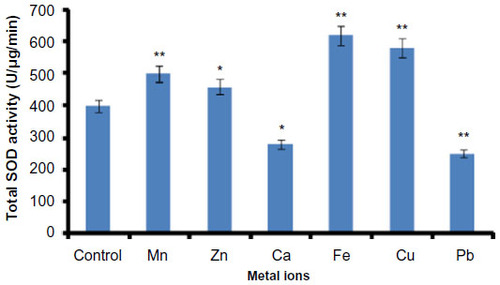
Figure 3 Identification of Helicoverpa armigera MnSOD.
Abbreviations: MnSOD, manganese SOD; SOD, superoxide dismutase.
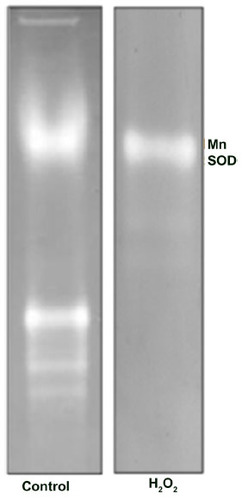
Table 1 Purification of Helicoverpa armigera MnSOD
Figure 4 Molecular weight determination and visualization of purified MnSOD.
Abbreviations: MnSOD, manganese superoxide dismutase; SDS, sodium dodecyl sulfate; CBBR-250, Coomassie Brilliant Blue R-250.
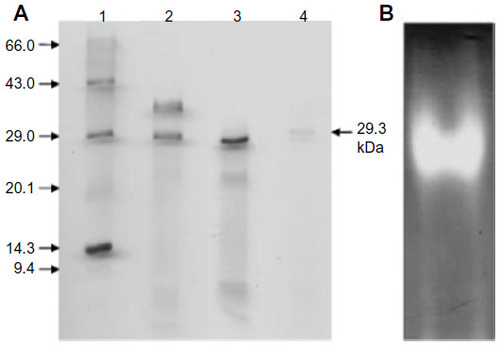
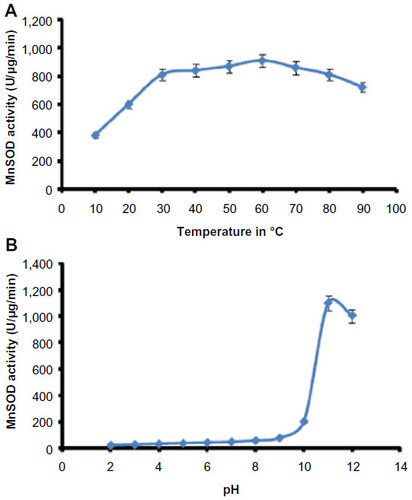
Figure 5 Optimum temperature and pH of Helicoverpa armigera MnSOD.
Abbreviations: MnSOD, manganese superoxide dismutase; SD, standard deviation.