Figures & data
Table 1 Baseline characteristics of subjects enrolled in studies of ethinylestradiol 20 µg/drospirenone 3 mg administered in a 24/4 treatment regimen conducted in the People’s Republic of China, Europe, Latin America, and the USA
Figure 1 Comparison of unadjusted Pearl Index scores in women after treatment over 1 year with the ethinylestradiol 20 µg/drospirenone 3 mg combined oral contraceptive (YAZ®) in three international studies conducted in the People’s Republic of China (Caiyan et alCitation18), Europe, Latin America, and the USA (Bachmann et alCitation10), and Europe (Hernádi et alCitation20).
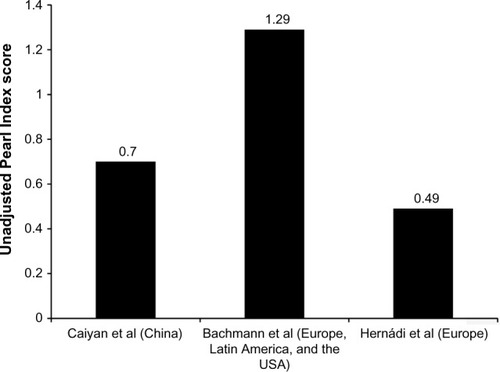
Figure 2 Percentage change from baseline to endpoint (defined as cycle 6 with last observation carried forward for dropouts) in mean total acne lesion count in women with moderate acne treated with ethinylestradiol 20 µg/drospirenone 3 mg combined oral contraceptive (YAZ®) or placebo in the studies conducted in the People’s Republic of China (Zhang et alCitation17) and the USA (Koltun et alCitation11 and Maloney et alCitation12). Values for the Koltun et al study are approximate as they were estimated from the graph presented in their publication. *P<0.001 versus placebo.
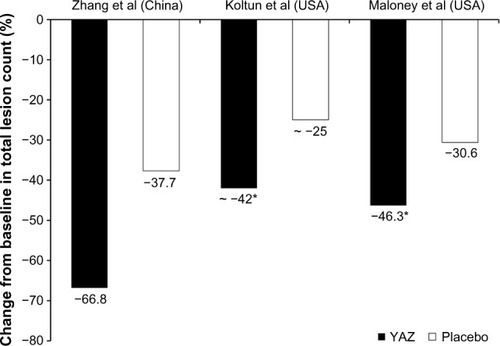
Figure 3 Change from baseline in DRSP scale scores after three cycles of treatment in women with premenstrual dysphoric disorder in ethinylestradiol 20 µg/drospirenone 3 mg combined oral contraceptive (YAZ®) placebo-controlled studies conducted in the People’s Republic of China (Fu et alCitation19) and the USA (Yonkers et alCitation14 and Pearlstein et alCitation13). *P<0.001 versus placebo.
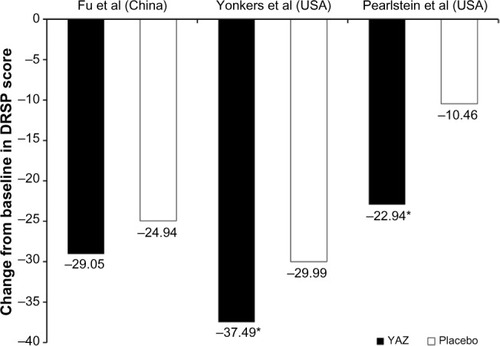
Table 2 Safety and tolerability of ethinylestradiol 20 µg/drospirenone 3 mg combined oral contraception administered in a 24/4 regimen (YAZ®) in women from the People’s Republic of China, Europe, Latin America, and the USA. Adverse events shown are those reported by ≥2% of participants
