Figures & data
Table 1 Overview Of Key Differences Between The Study Designs Of OT-0401, REVERSE, And CONFIRM
Figure 1 CONFIRM study design. Copyright © 2012. Dove Medical Press. Adapted from Boyer TD, Medicis JJ, Pappas SC, Potenziano J, Jamil K. A randomized, placebo-controlled, double-blind study to confirm the reversal of hepatorenal syndrome type 1 with terlipressin: the REVERSE trial design. Open Access J Clin Trials. 2012;4:39–49.Citation16
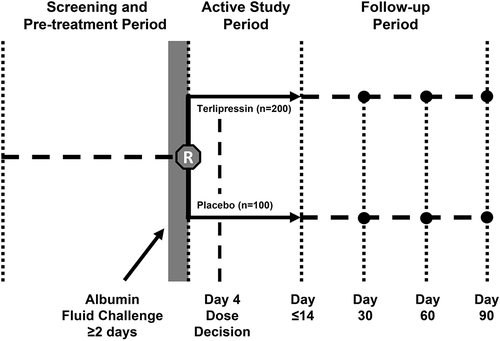
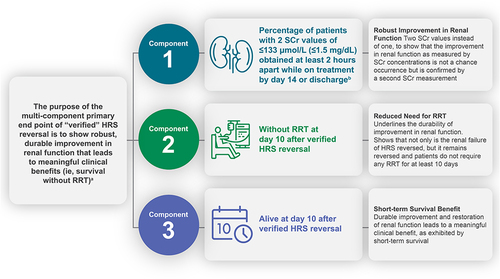
Figure 2 Overview of the multi-component primary end point in CONFIRM. aVerified reversal is assessed as percentage of patients achieving this end point on terlipressin vs placebo; open-label albumin is administered in both treatment groups. bTreatment period is up to Day 14 or discharge, whichever comes first.