Figures & data
Table 1 Pattern of changes in cumulative dose in the between-patient three-level response surface pathway design
Figure 1 Dose escalation and de-escalation procedures in a between-patient and a within-patient response surface pathway design.
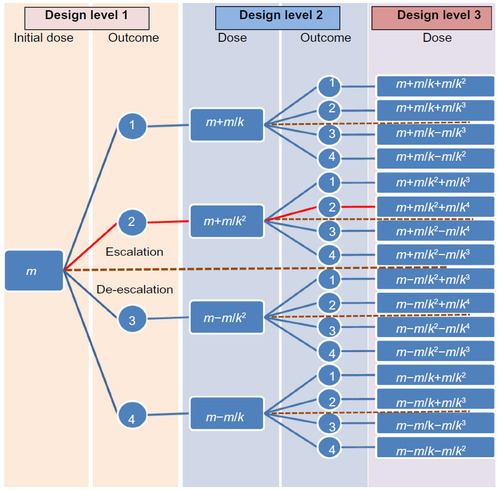
Figure 2 The pathway when estimating the maximum tolerated dose of BP-C1 in women with stage IV breast cancer using a between-patient response surface pathway design.
Abbreviation: BW, body weight.
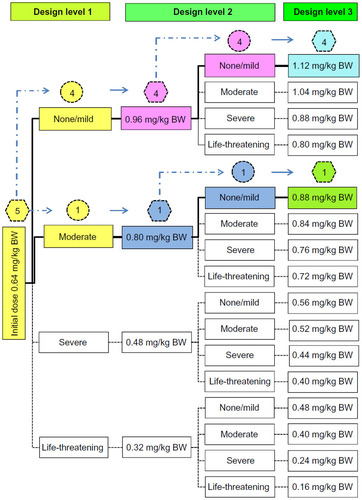
Figure 3 The pathway when estimating the maximum tolerated dose of BP-C1 in dogs with metastatic mammary cancer using a within-patient response surface pathway design.
Abbreviation: BW, body weight.
Table 2 Performance of the traditional 3+3 design and response surface pathway design in three scenarios
