Figures & data
Figure 1 Patient disposition flow chart showing the total number of patients randomized to each study (by treatment group), as well as the number of individuals that completed each study (by treatment group).
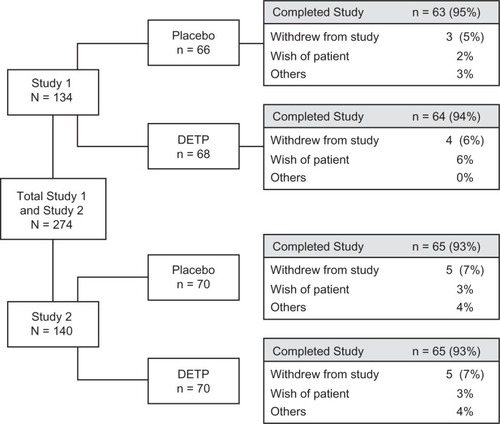
Table 1 Patient demographics and other baseline characteristics
Figure 2 Percent (%) reduction of post-treatment scores for pain on movement (visual analog scale; VAS) in patients treated with diclofenac epolamine topical patch (DETP) and placebo. Pain reduction was statistically significantly different between treatment groups, favoring DETP treatment, starting 3 hours after initial patch application and continuing through the end of the study (day 7). The asterisk denotes statistical significance (P ≤ 0.05).
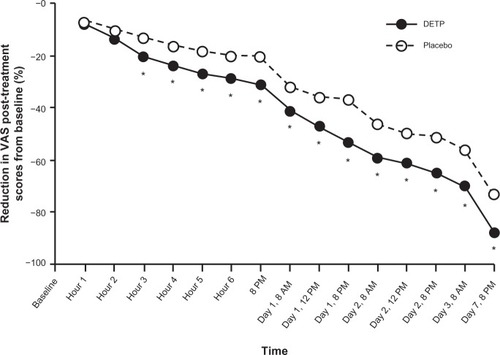
Table 2 Pooled scores by treatment group for pain on movement: self-assessed by patients on 100 mm VAS (0 = no pain to 100 = severe pain) from the first day of treatment (day 0) through day 7 of the study (day 7)
Table 3 Secondary analgesic efficacy results: individual data from Study 1 and Study 2
Figure 3 Global efficacy assessments for each treatment group judged by both patients and investigators at day 7 for Study 1 A) and Study 2 B). This assessment revealed that, for both studies, the diclofenac epolamine topical patch (DETP) treatment was superior to placebo treatment at day 7; day 3 analyses demonstrated comparable results (not shown).
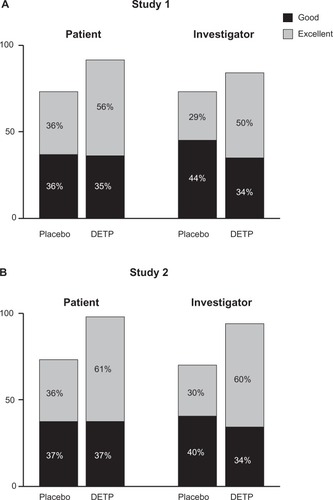
Figure 4 Overall tolerability of diclofenac epolamine topical patch (DETP) or placebo for each treatment group, assessed by patients and investigators at day 7 for Study 1 A) and Study 2 B). The majority of patients in both treatment groups judged the patch tolerability as excellent. No significant differences were found between DETP- and placebo-treated patients at day 7; results from day 3 tolerability assessments were comparable (not shown).
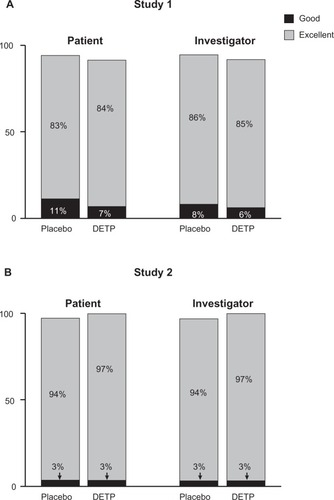
Table 4 Summary of adverse events reported during Study 1