Figures & data
Table 1 Demographics and Baseline Disease Characteristics for Patients Receiving Tofacitinib Monotherapy in Clinical Trials and Available RWD Sources
Figure 1 Rates of achieving (A) CDAI-defined LDA (≤10), (B) CDAI-defined remission (≤2.8), (C) DAS28-4(ESR)-defined LDA (≤3.2), and (D) DAS28-4(ESR)-defined remission (<2.6) in patients receiving tofacitinib monotherapy in clinical trialsa and available RWD sources.
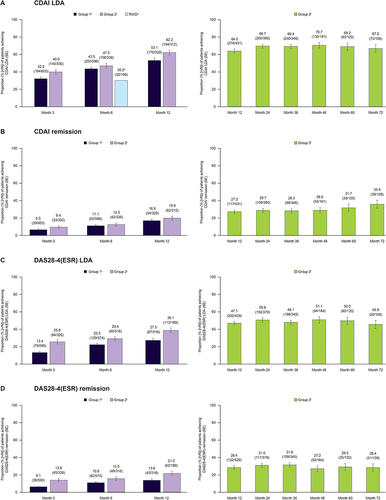
Figure 2 Rates of achieving (A) DAS28-4(CRP)-defined LDA (≤3.2)a and (B) DAS28-4(CRP)-defined remission (<2.6)a in patients receiving tofacitinib monotherapy in clinical trialsb and available RWD sources.
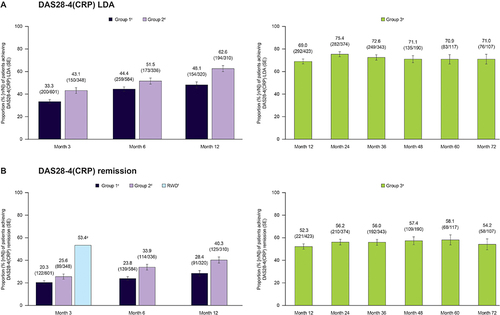
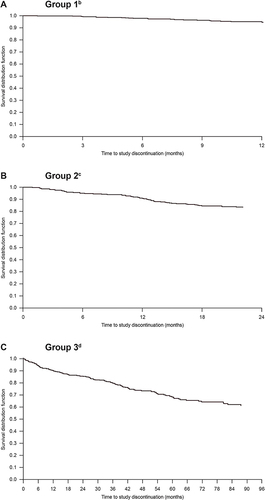
Figure 3 Kaplan–Meier plots of time to discontinuation of tofacitinib 5 mg Bid monotherapy due to lack of efficacy or adverse events in clinical trialsa: (A) Group 1, (B) Group 2, and (C) Group 3.
Data Sharing Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
