Figures & data
Figure 1 The efficacy of aflibercept for treating AMD resistant to ranibizumab (patient 3 with occult CNV).
Abbreviations: AMD, age-related macular degeneration; CNV, choroidal neovascularization; IVA, intravitreal aflibercept; IVR, intravitreal ranibizumab; PED, pigment epithelial detachment; SD-OCT, spectral domain optical coherence tomography; SRF, subretinal fluid.
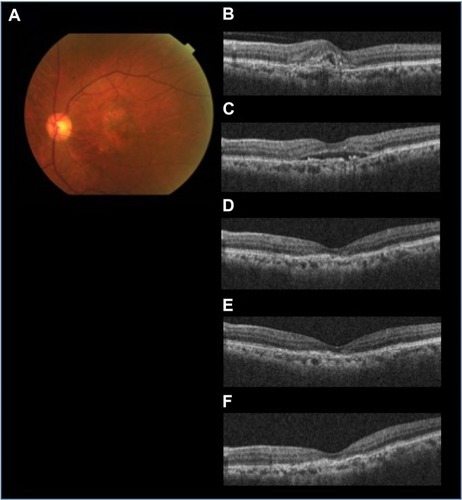
Figure 2 The efficacy of aflibercept for AMD refractory to ranibizumab (patient 8 with PCV).
Abbreviations: AMD, age-related macular degeneration; IVA, intravitreal aflibercept; IVR, intravitreal ranibizumab; PCV, polypoidal choroidal vasculopathy; PED, pigment epithelial detachment; SD-OCT, spectral domain optical coherence tomography; SRF, subretinal fluid.
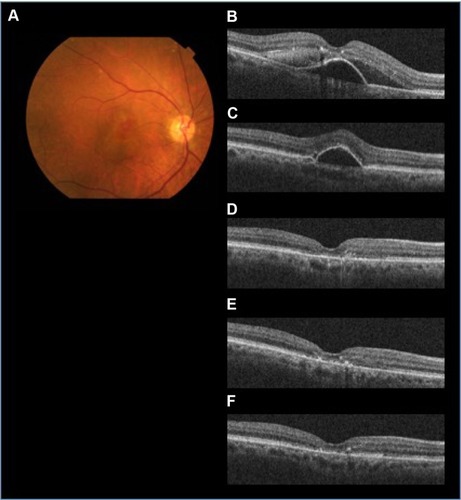
Table 1 Demographics of 14 patients with AMD
Figure 3 The efficacy of aflibercept assessed based on the visual acuity and central macular thickness in 14 patients with AMD (seven patients with occult CNV and seven subjects with PCV).
Abbreviations: AMD, age-related macular degeneration; CNV, choroidal neovascularization; IVA, intravitreal aflibercept; logMAR, logarithm of minimum angle of resolution; PCV, polypoidal choroidal vasculopathy; SD-OCT, spectral domain optical coherence tomography.
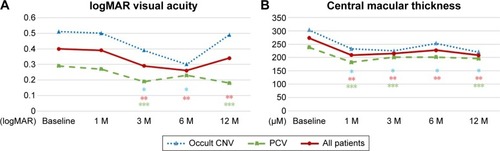
Table 2 Best-corrected visual acuity of 14 patients treated with IVA injection
Table 3 Central macular thickness of 14 patients treated with IVA
Table 4 Anatomical response to IVA treatment in 14 patients with AMD
