Figures & data
Figure 1 Device A osmometer readings vs ideal dilution osmolarity.
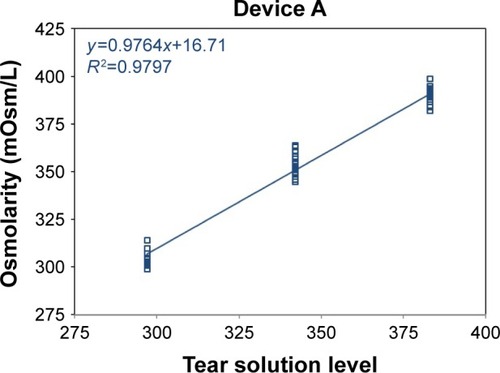
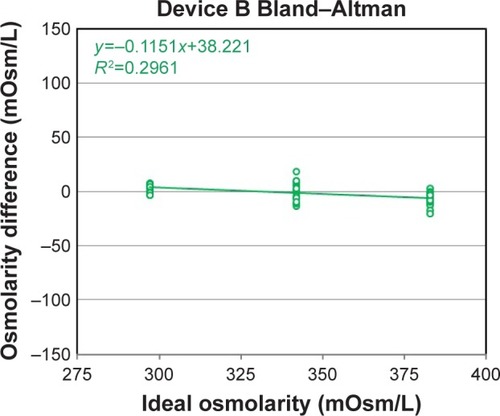
Figure 2 Device B osmometer readings vs ideal dilution osmolarity.
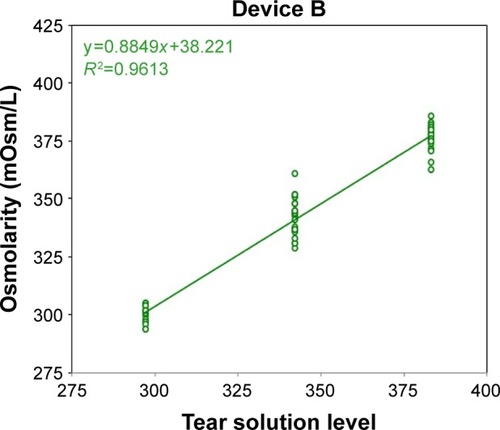
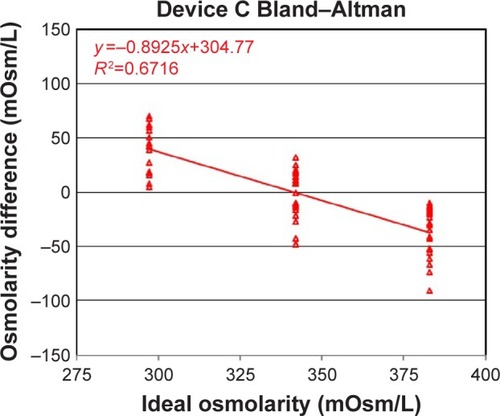
Figure 3 Device C osmometer readings vs ideal dilution osmolarity.
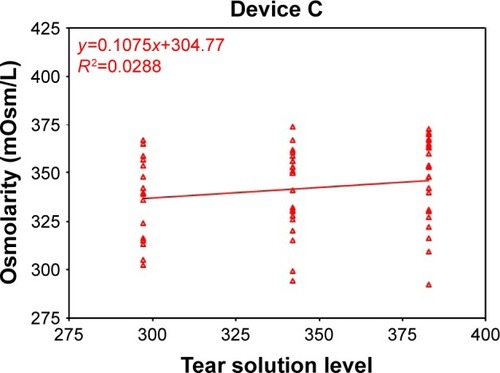
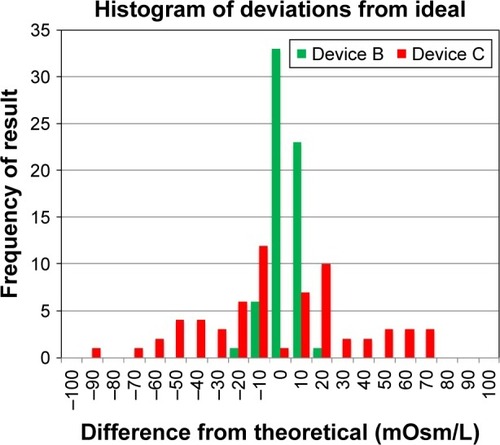
Figure 4 Device B and device C osmometers vs device A measured osmolarity.
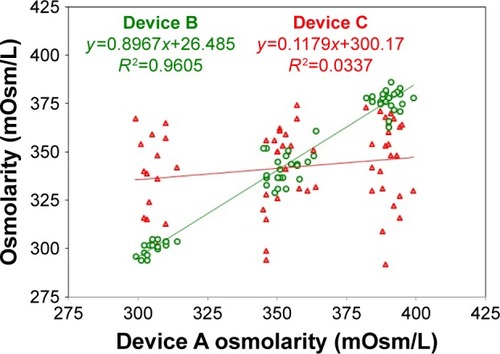
Table 1 Combined osmolarity data on contrived tear solutions for device A, device B, and device C osmometers