Figures & data
Table 1 Treatment regimens before and after initiating treatment with the preservative-free fixed combination tafluprost 0.0015%/timolol 0.5%
Table 2 Patient characteristics of the patient population (N=1,157) included in the observational study with the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5%
Figure 1 Reasons for changing medication to preservative-free tafluprost/timolol fixed combination.
Abbreviation: IOP, intraocular pressure.
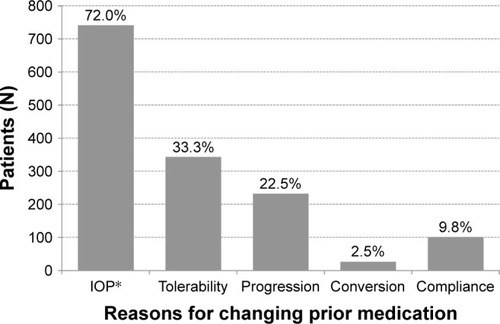
Figure 2 IOP at baseline and at final visit in each individual patient irrespective of prior treatment with the preservative-free tafluprost/timolol fixed combination as the only medication at final visit (N=1,075).
Abbreviation: IOP, intraocular pressure.
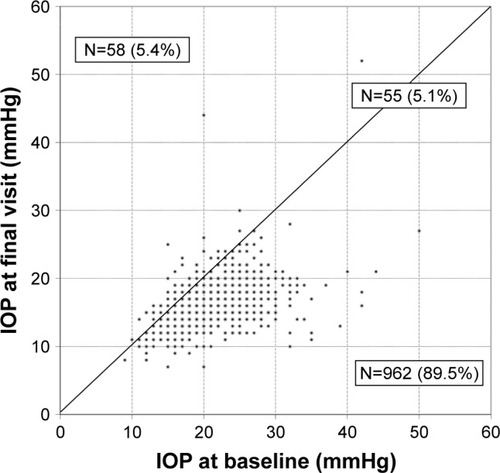
Figure 3 Decrease of mean IOP (±SD) for different subgroups: Naïve patients (N=127) and patients with prior monotherapy with beta-blockers (N=163), PGAs (N=338), carbonic anhydrase inhibitors (N=48) and alpha-2-agonists (N=28) treated with the preservative-free tafluprost/timolol fixed combination as the only medication at final visit.
Abbreviations: CAIs, carbonic anhydrase inhibitors; IOP, intraocular pressure; PGAs, prostaglandin analogs; SD, standard deviation.
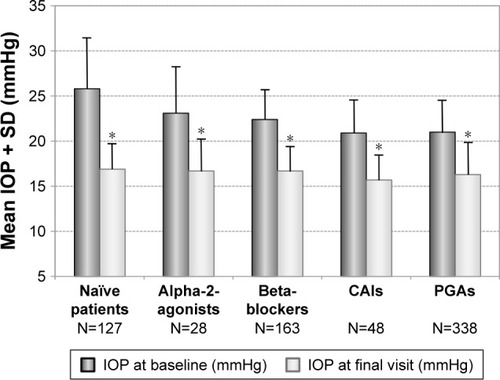
Figure 4 Decrease of mean IOP (±SD) in patients with prior monotherapy with different prostaglandin analogs: preserved latanoprost (Lat P; N=113), preservative-free latanoprost (Lat PF; N=59), preserved bimatoprost (Bim P; N=53), preserved travoprost (Tra P; N=40), preserved tafluprost (Taf P; N=34) and preservative-free tafluprost (Taf PF; N=37) treated with preservative-free tafluprost/timolol fixed combination as the only medication at final visit.
Abbreviations: IOP, intraocular pressure; SD, standard deviation.
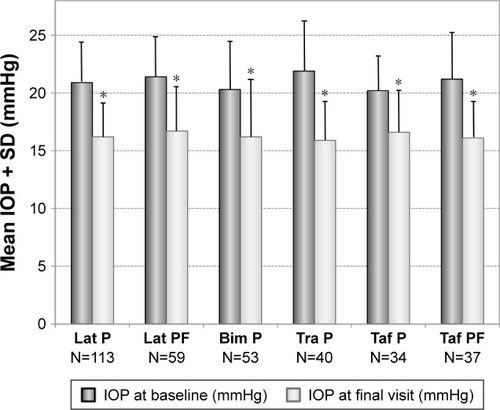
Figure 5 Decrease of mean IOP (±SD) for different subgroups with prior fixed combinations: PGA/timolol (N=163), carbonic anhydrase inhibitor/timolol (N=66) and other fixed combinations (N=9) treated with preservative-free tafluprost/timolol fixed combination as the only medication at final visit.
Abbreviations: CAI, carbonic anhydrase inhibitor; FC, fixed combination; IOP, intra-ocular pressure; PGA, prostaglandin analog; SD, standard deviation; TIM, timolol.
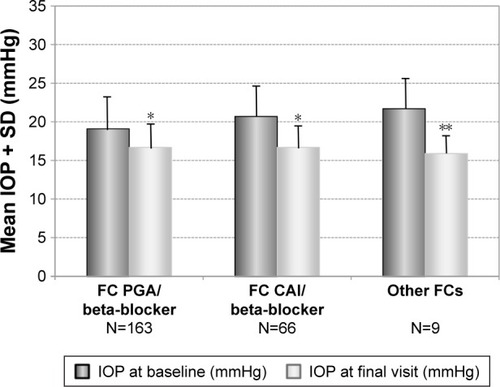
Figure 6 Decrease of mean IOP (±SD) in patients with prior medical therapy with different preserved prostaglandin analog/timolol fixed combinations: latanoprost/timolol (N=47), bimatoprost/timolol (N=73) and travoprost/timolol (N=36) treated with the preservative-free tafluprost/timolol fixed combination as the only medication at final visit.
Abbreviations: BIM, bimatoprost; FC, fixed combination; IOP, intraocular pressure; LAT, latanoprost; SD, standard deviation; TRA, travoprost; TIM, timolol.
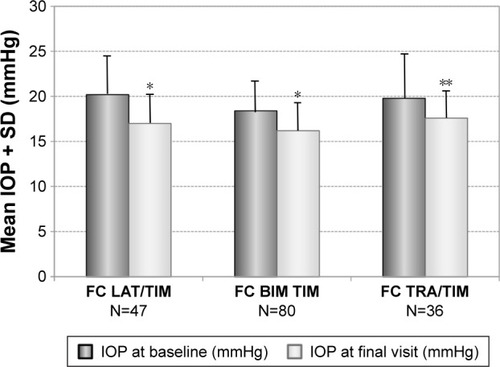
Figure 7 Decrease of mean IOP (±SD) for different subgroups with prior non-fixed combinations: PGA + beta-blocker (N=55), PGA + carbonic anhydrase inhibitor (N=18) and nonfixed combinations of three different products (N=35) treated with the preservative-free tafluprost/timolol fixed combination as the only medication at final visit.
Abbreviations: CAI, carbonic anhydrase inhibitor; IOP, intraocular pressure; NFC, non-fixed combination; PGA, prostaglandin analog; SD, standard deviation.
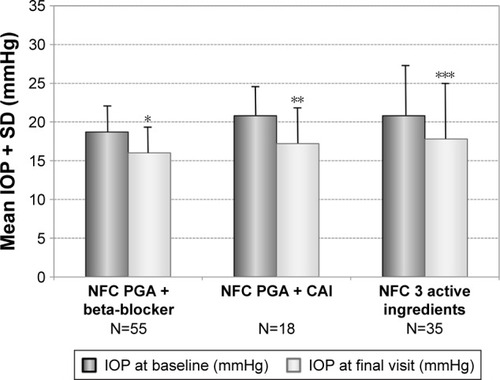
Figure 8 Decrease of IOP (mmHg) from different baseline IOP levels in treatment-naïve patients after initiation of medical treatment with the preservative-free tafluprost/timolol fixed combination (N=127).
Abbreviation: IOP, intraocular pressure.
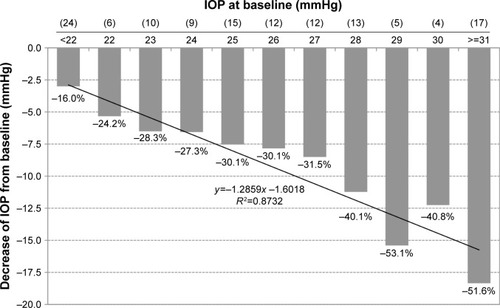
Figure 9 Conjunctival hyperemia at baseline and after initiation of medical therapy with or change of medication in patients treated with the preservative-free tafluprost/timolol fixed combination as the only medication at final visit: All patients with prior medical therapy (N=948), naïve patients (N=127), patients with prior PGA monotherapy (N=338), patients with prior fixed combination PGA/timolol (N=163) and patients with prior non-fixed combinations of a PGA and timolol (N=55).
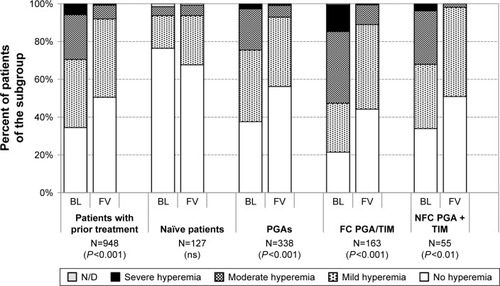
Figure 10 Conjunctival staining at baseline and after initiation of medical therapy with or change of medication in patients treated with preservative-free tafluprost/timolol fixed combination as the only medication at final visit: All patients with prior medical therapy (N=948), naïve patients (N=127), patients with prior PGA monotherapy (N=338), patients with prior fixed combination PGA/timolol (N=163) and patients with prior nonfixed combination of a PGA and timolol (N=55).
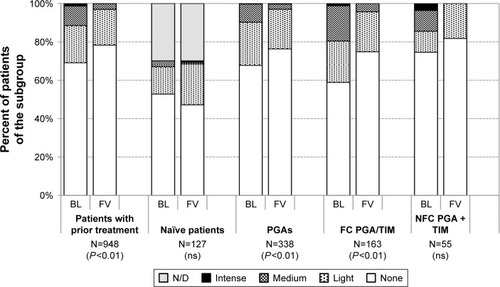
Figure 11 Grading of lid-parallel conjunctival folds at baseline and after initiation of medical therapy with or change of medication in patients treated with preservative-free tafluprost/timolol fixed combination as the only medication at final visit: All patients with prior medical therapy (N=948), naïve patients (N=127), patients with prior PGA monotherapy (N=338), patients with prior fixed combination PGA/timolol (N=163) and patients with prior nonfixed combination of a PGA and timolol (N=55).
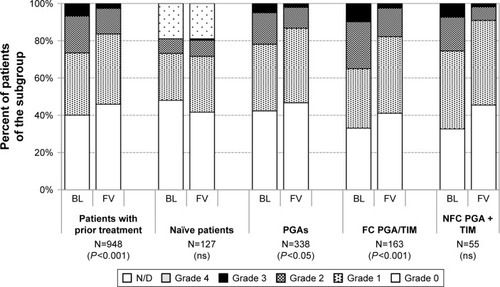
Figure 12 Severity of ocular symptoms in patients with prior medical treatment at baseline and after change of medication to the preservative-free tafluprost/timolol fixed combination (N=948).
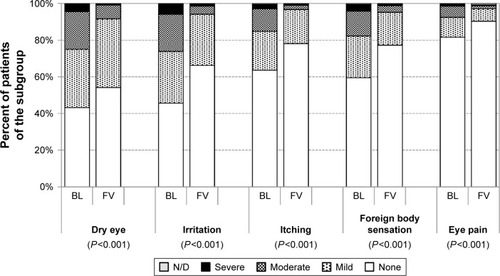
Figure 13 Severity of ocular symptoms in treatment naïve patients at baseline and at final visit after initiation of medical treatment with the preservative-free tafluprost/timolol fixed combination (N=127).
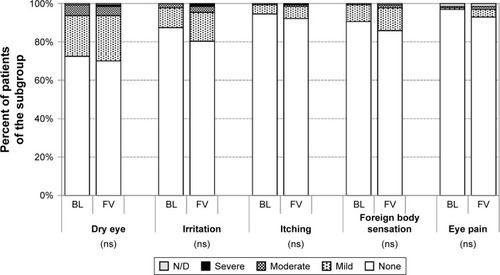
Table 3 Adverse events and terminations of treatment for the overall study population (N=1,157)
