Figures & data
Figure 1 RT-PCR products of LTA mRNA detected by 3% agarose gel electrophoresis stained by ethidium bromide.
Abbreviations: RT-PCR, reverse transcription-polymerase chain reaction; LTA, lymphotoxin alpha; mRNA, messenger RNA.
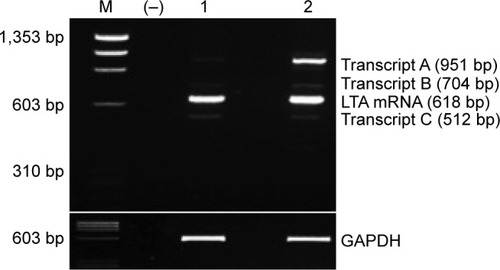
Figure 2 Expression analysis of T and C alleles of LTA rs2229094 polymorphism.
Abbreviations: LTA, lymphotoxin alpha; RT-PCR, reverse transcription-polymerase chain reaction.

Figure 3 Subcellular localization of T and C alleles of LTA rs2229094 polymorphism.
Abbreviations: LTA, lymphotoxin alpha; DAPI, 4′,6-diamidino-2-phenylindole.
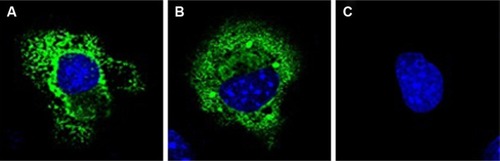
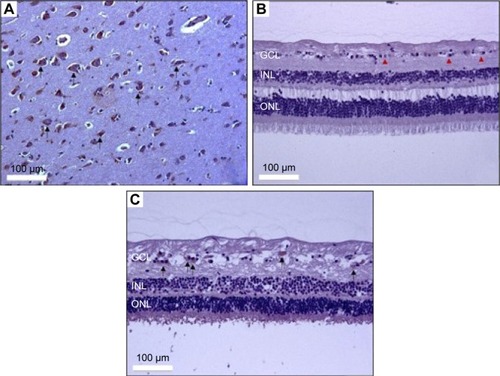
Figure 4 Immunohistochemical assay of LTA in human retina.
Abbreviations: LTA, lymphotoxin alpha; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.