Figures & data
Figure 1 Follow-up over 12 months in nAMD, DME, and BRVO patients.
Abbreviations: nAMD, neovascular age-related macular degeneration; DME, diabetic macular edema; BRVO, branch retinal vein occlusion.
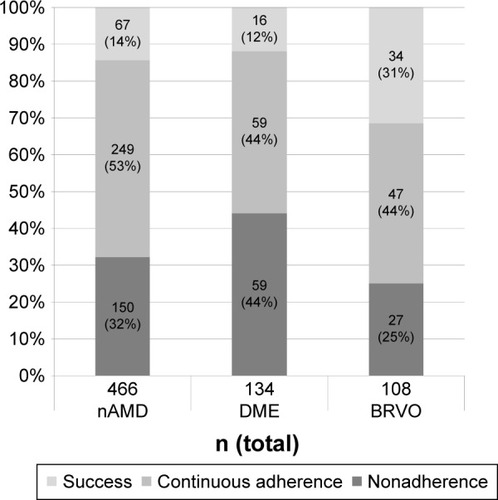
Figure 2 Reasons for unintended treatment gaps (NA).
Abbreviations: nAMD, neovascular age-related macular degeneration; DME, diabetic macular edema; BRVO, branch retinal vein occlusion; NA, nonadherence.
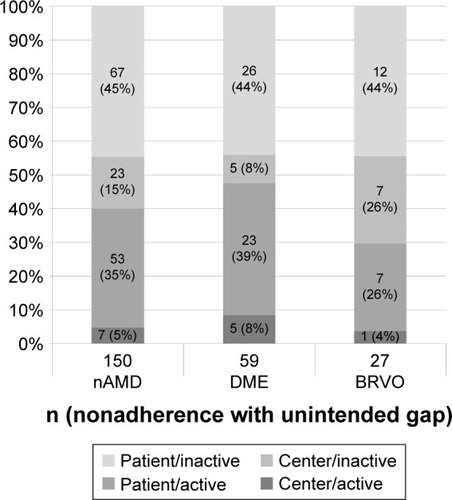
Figure 3 Kaplan–Meier estimation of incidence of NA over the course of 365 days.
Abbreviations: NA, nonadherence; nAMD, neovascular age-related macular degeneration; DME, diabetic macular edema; BRVO, branch retinal vein occlusion.
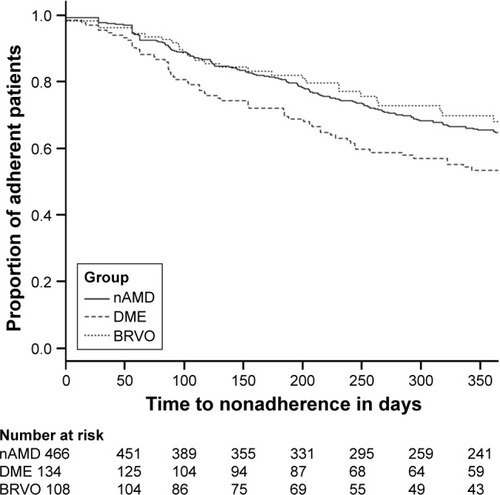
Table 1 Regression analysis for risk factors for patient-associated NA
Figure 4 Development of visual acuity after 12 months for nAMD, DME, and BRVO, stratified for follow-up (adherent/nonadherent).
Abbreviations: nAMD, neovascular age-related macular degeneration; DME, diabetic macular edema; BRVO, branch retinal vein occlusion.
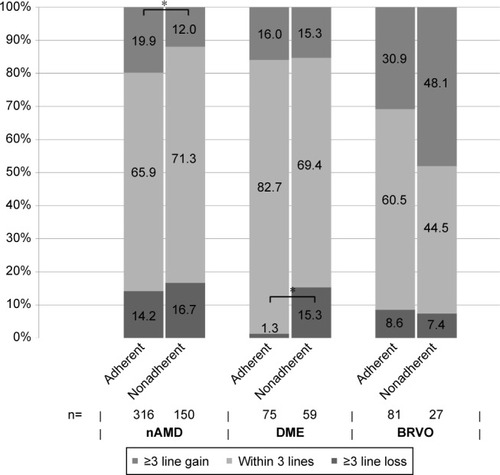
Table 2 Description of study groups with patient and treatment characteristics, stratified for adherence
