Figures & data
Figure 1 Study design of OPUS-3.
Abbreviations: AE, adverse event; BID, twice daily.
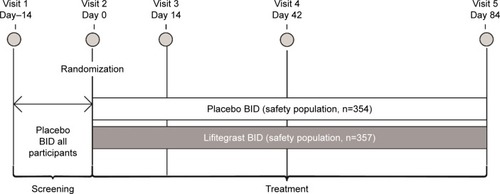
Table 1 Demographic and baseline characteristics (randomized population)
Figure 2 Drop comfort scores at 0–3 minutes postinstillation (safety population).
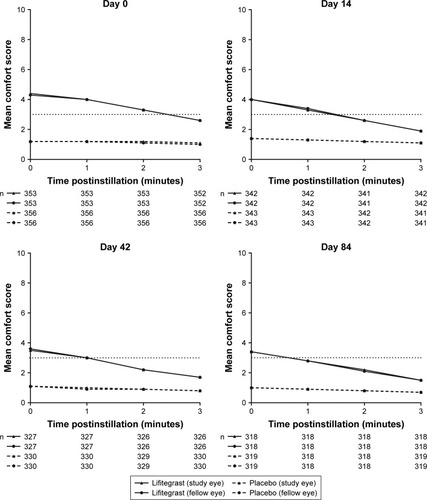
Figure 3 Participants with drop comfort score <3 by visit (both eyes; safety population).
Abbreviations: LIF, lifitegrast; PBO, placebo.
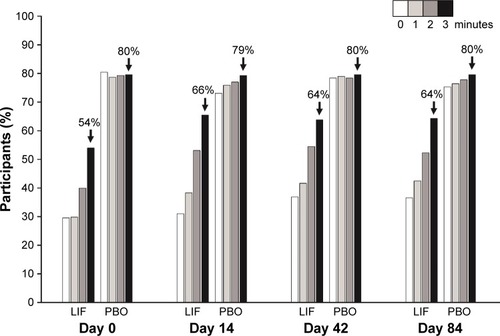
Figure 4 Drop comfort scores at 5, 10, and 15 minutes postinstillation among participants with drop comfort score >3 at 3-minute assessment (safety population; numbers above bars indicate participant numbers).
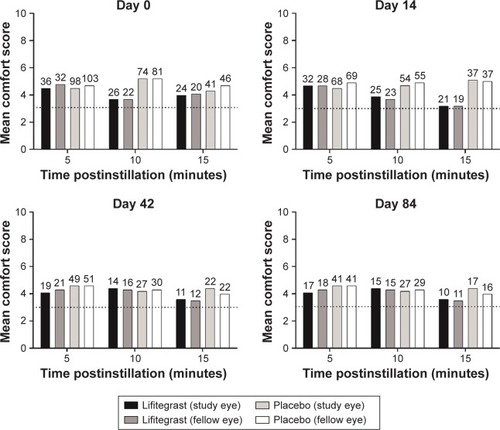
Table 2 Demographics and baseline characteristics of participants with drop comfort score >3 in the study eye at ≥15 minutes postinstillation (safety population)
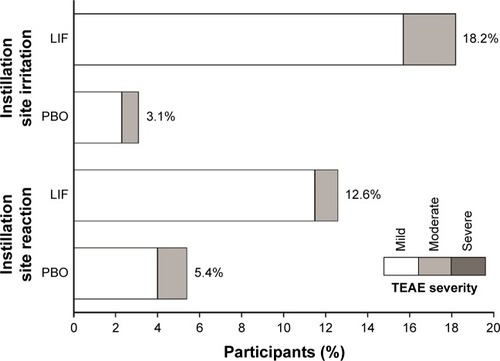
Figure 5 Incidence and severity of common ocular TEAEs (occurring in >5% in either treatment group; safety population).
Abbreviations: LIF, lifitegrast; PBO, placebo; TEAE, treatment-emergent adverse event.