Figures & data
Figure 1 The UltraSert® preloaded delivery system.
Abbreviation: IOL, intraocular lens.
Table 1 Study population demographics and baseline characteristics
Figure 2 Mean corneal incision size after intraocular lens implantation of preloaded systems or manual delivery system.
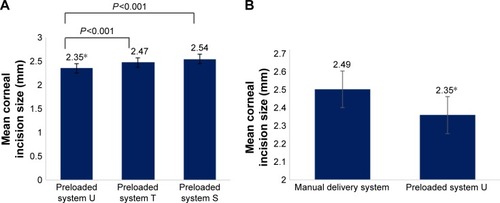
Figure 3 Mean corneal incision size enlargement caused by IOL delivery using preloaded systems or manual delivery system.
Abbreviation: IOL, intraocular lens.
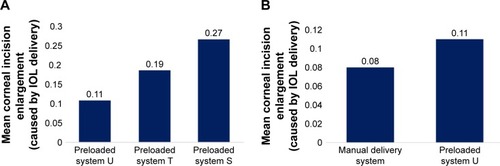
Figure 4 SIA following implantation of preloaded systems or manual delivery system.
Abbreviations: D, diopters; SIA, surgically induced astigmatism.
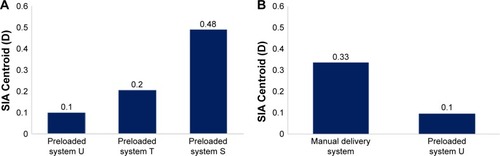
Table 2 Observations of IOL–delivery device interactions and surgically induced astigmatism
Table 3 Treatment emergent ocular adverse events (serious and nonserious)
