Figures & data
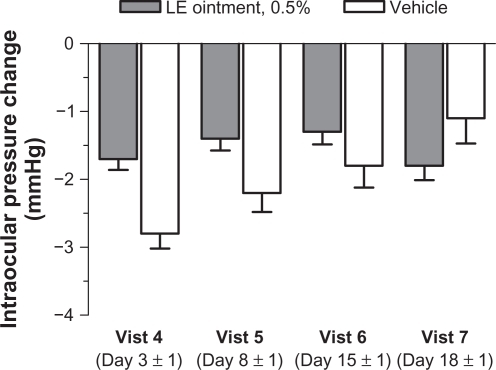
Figure 1 Flow of patients through the study.
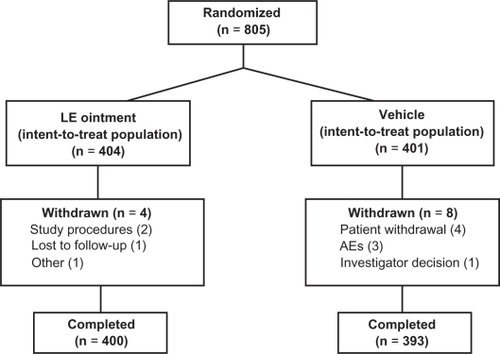
Table 1 Demographics, intent-to-treat population
Figure 2 Proportion of patients with rescue medication use prior to each visit.
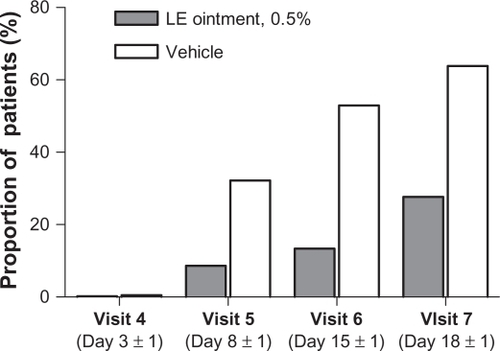
Table 2 Resolution of anterior chamber cells and flare and pain at visit 5: integrated intent-to-treat population
Figure 3 Proportion of patients with complete resolution of anterior chamber inflammation A), complete resolution of anterior chamber cells B), complete resolution of anterior chamber flare C), and no (Grade 0) pain D) at each study visit.
Abbreviation: LE, loteprednol etabonate.
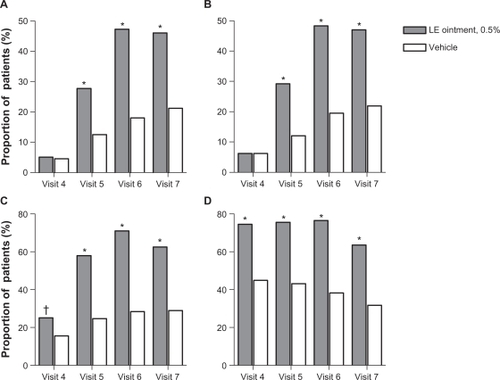
Table 3 Ocular adverse events occurring in ≥3% study eyes in any treatment group prior to rescue medication