Figures & data
Table 1 Patient demographic characteristics for the first observed nAMD encountersTable Footnotea
Figure 1 Rate of endophthalmitis in nAMD patients.
Abbreviation: nAMD, neovascular age-related macular degeneration.
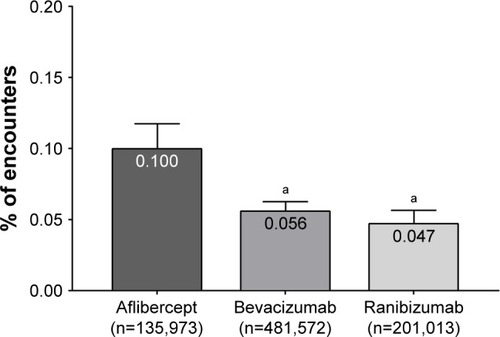
Table 2 Repeated-measures analysis for risk of endophthalmitis among nAMD patient encounters (N=818,558)
Figure 2 Rate of endophthalmitis during each 6-month period of the 18-month study in nAMD patients.
Abbreviation: nAMD, neovascular age-related macular degeneration.
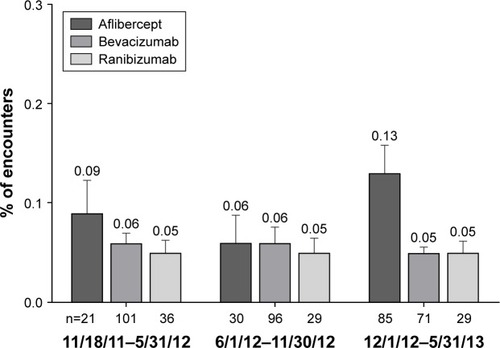
Figure 3 Days from anti-VEGF treatment to first encounter of endophthalmitis for nAMD patients.
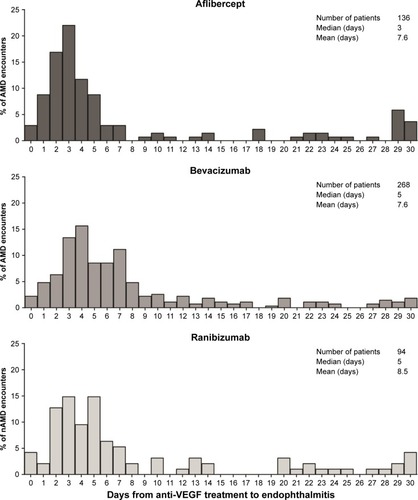
Figure S1 Patient encounter identification.
Note: aData for RVO and DME are not reported here because the sample size for the RVO analysis for aflibercept was small compared with those for ranibizumab and bevacizumab, and aflibercept was not approved for treatment of DME at the time of the study.
Abbreviations: DME, diabetic macular edema; nAMD, neovascular age-related macular degeneration; RVO, retinal vein occlusion; VEGF, vascular endothelial growth factor.
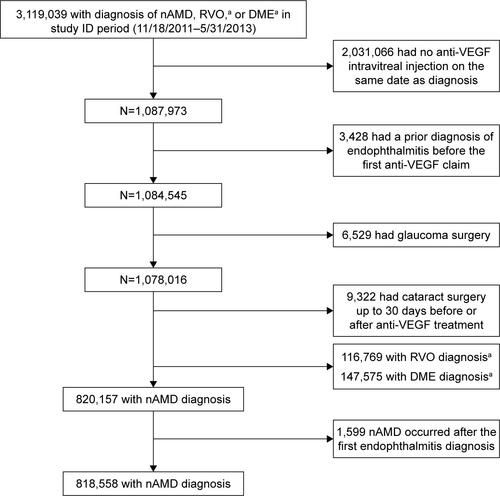
Figure S2 Rate of endophthalmitis by injection order in patients with nAMD.
Abbreviation: nAMD, neovascular age-related macular degeneration.
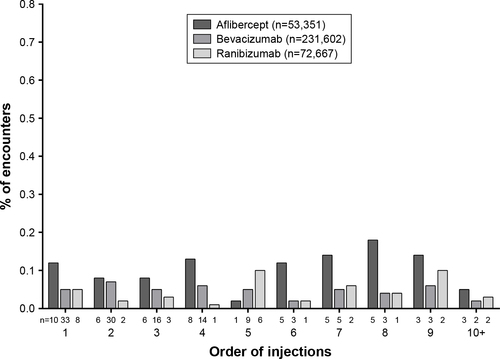
Figure S3 Injectable antibiotic treatment within 14 days after endophthalmitis for nAMD patient encounters.
Abbreviation: nAMD, neovascular age-related macular degeneration.
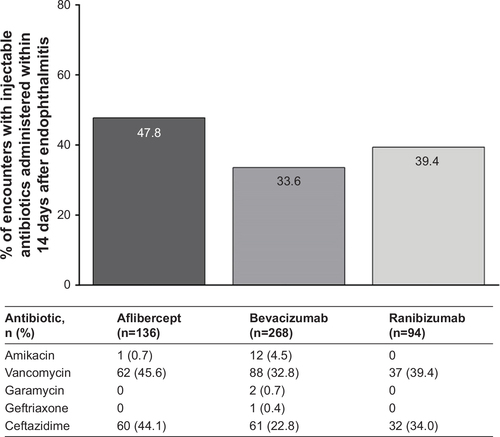
Figure S4 Management of incidences of endophthalmitis using vitrectomy for nAMD patient encounters.
Abbreviation: nAMD, neovascular age-related macular degeneration.
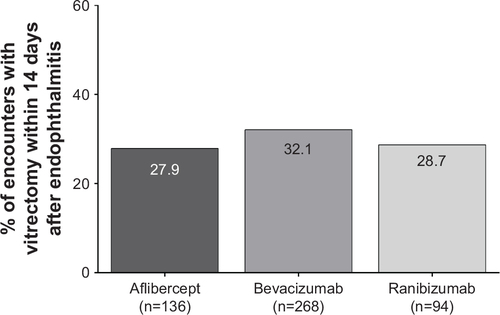
Figure S5 Anti-VEGF use within 6 months of endophthalmitis from nAMD patient encounters.
Notes: aEver used a different anti-VEGF agent within 6 months. bUsed the same anti-VEGF agent within 6 months.
Abbreviations: nAMD, neovascular age-related macular degeneration; VEGF, vascular endothelial growth factor.
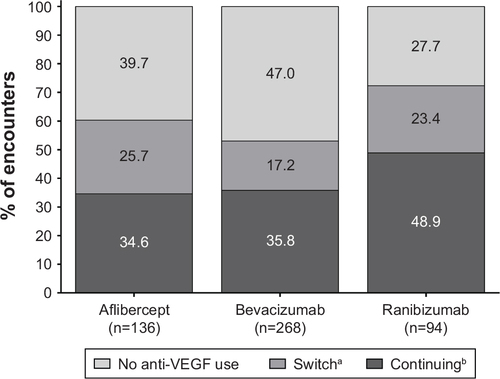
Table S1 ICD-9-CM codes for identifying neovascular age-related macular degeneration and endophthalmitis
Table S2 Healthcare Common Procedure Coding System codes
